Search
- Page Path
-
- HOME
- Search
-

-

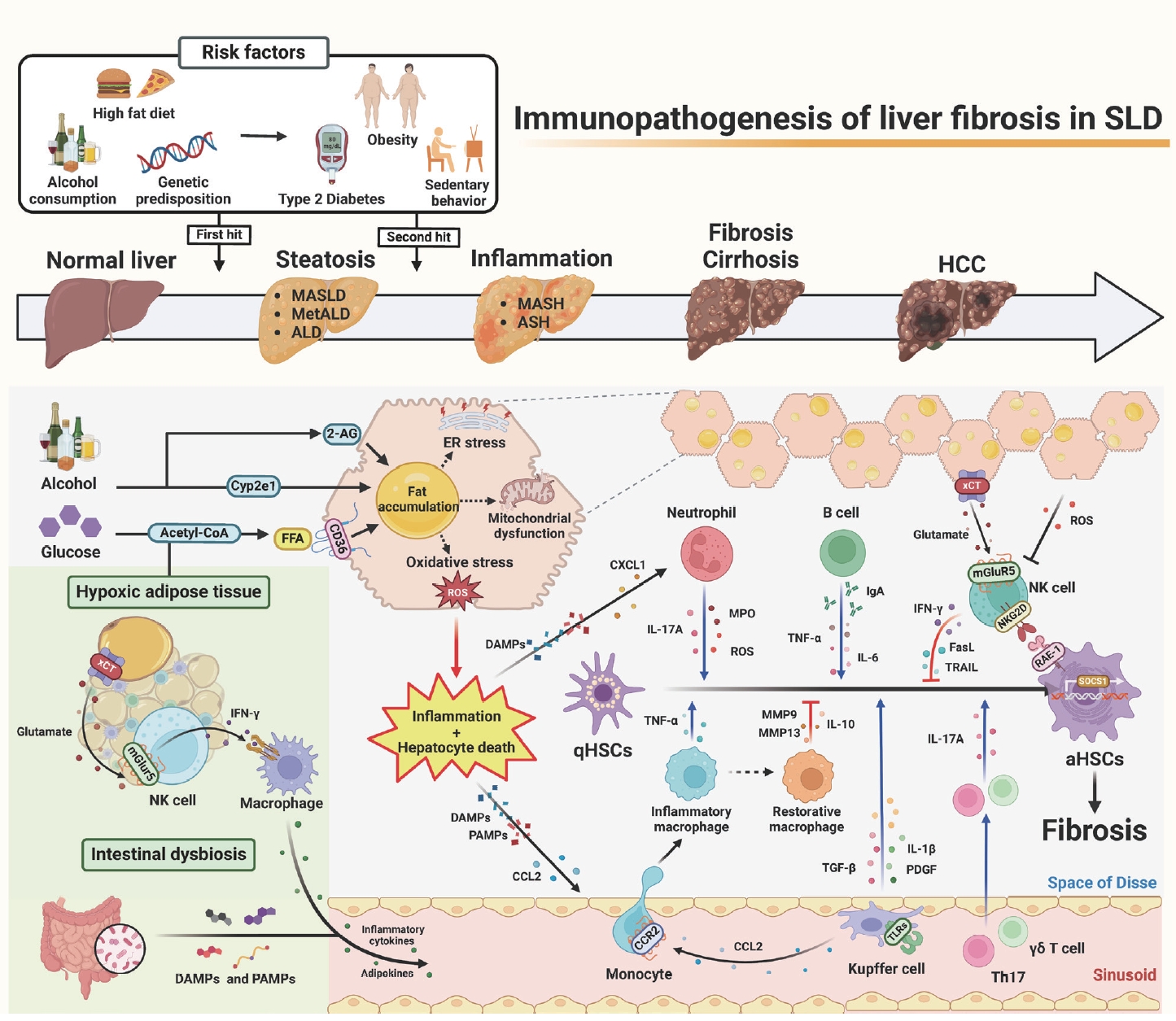
- Immunopathogenesis of liver fibrosis in steatotic liver disease
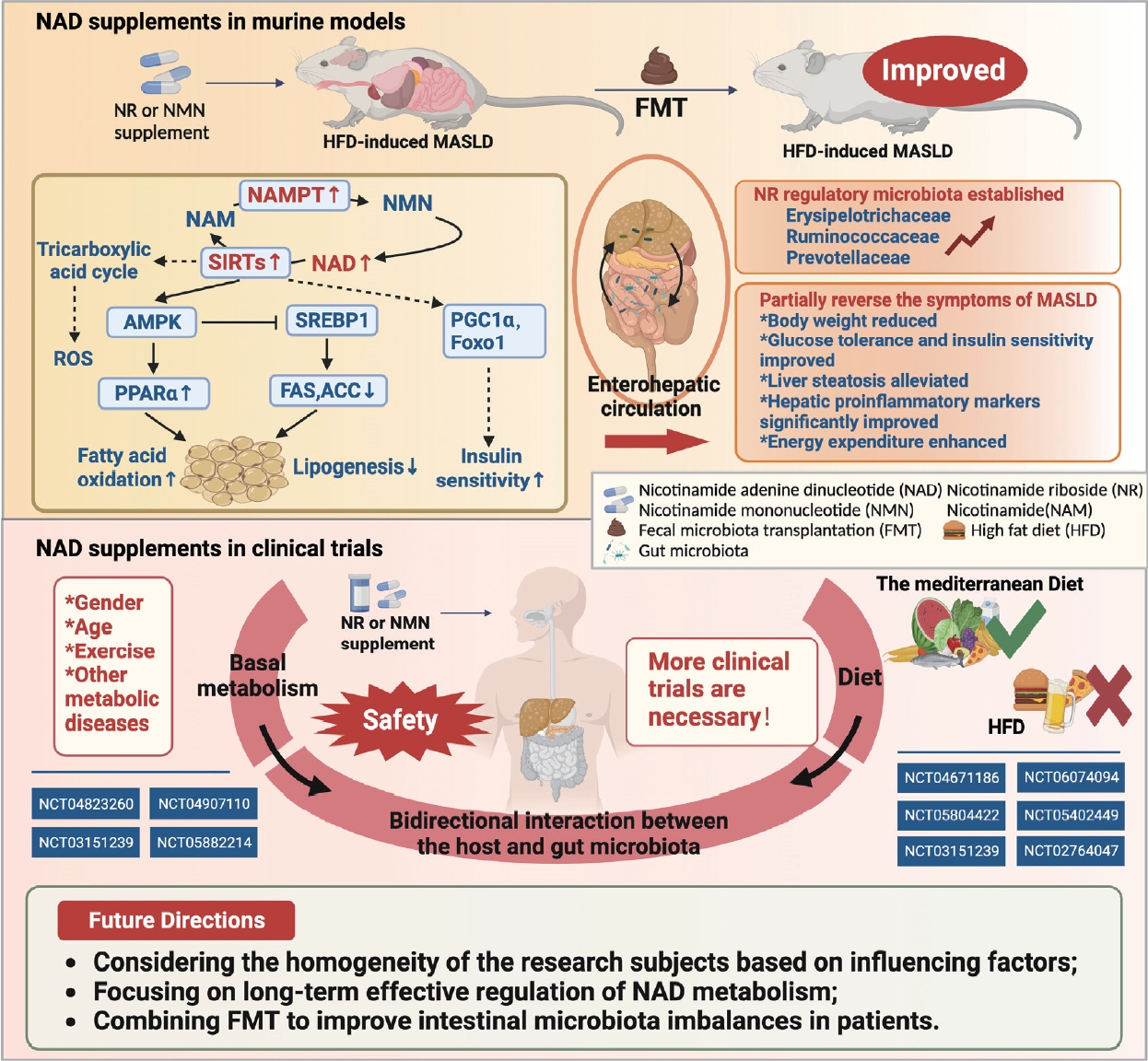
- Evaluating the therapeutic efficacy of NAD supplementation in management of metabolic dysfunction-associated steatotic liver disease: Key considerations
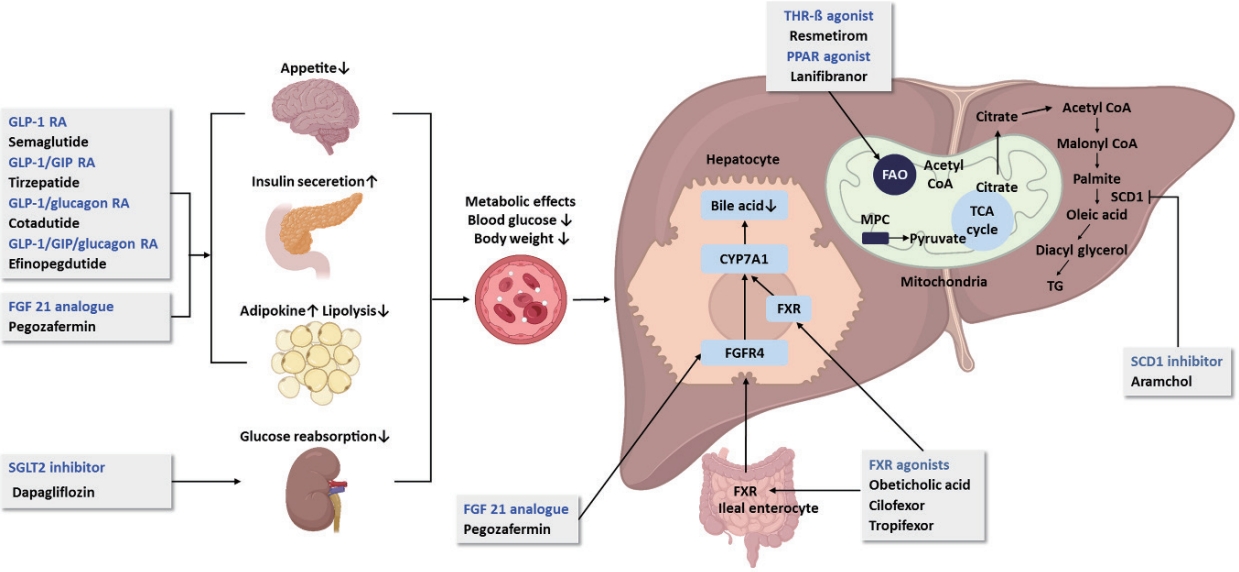
- Recent updates on pharmacologic therapy in non-alcoholic fatty liver disease
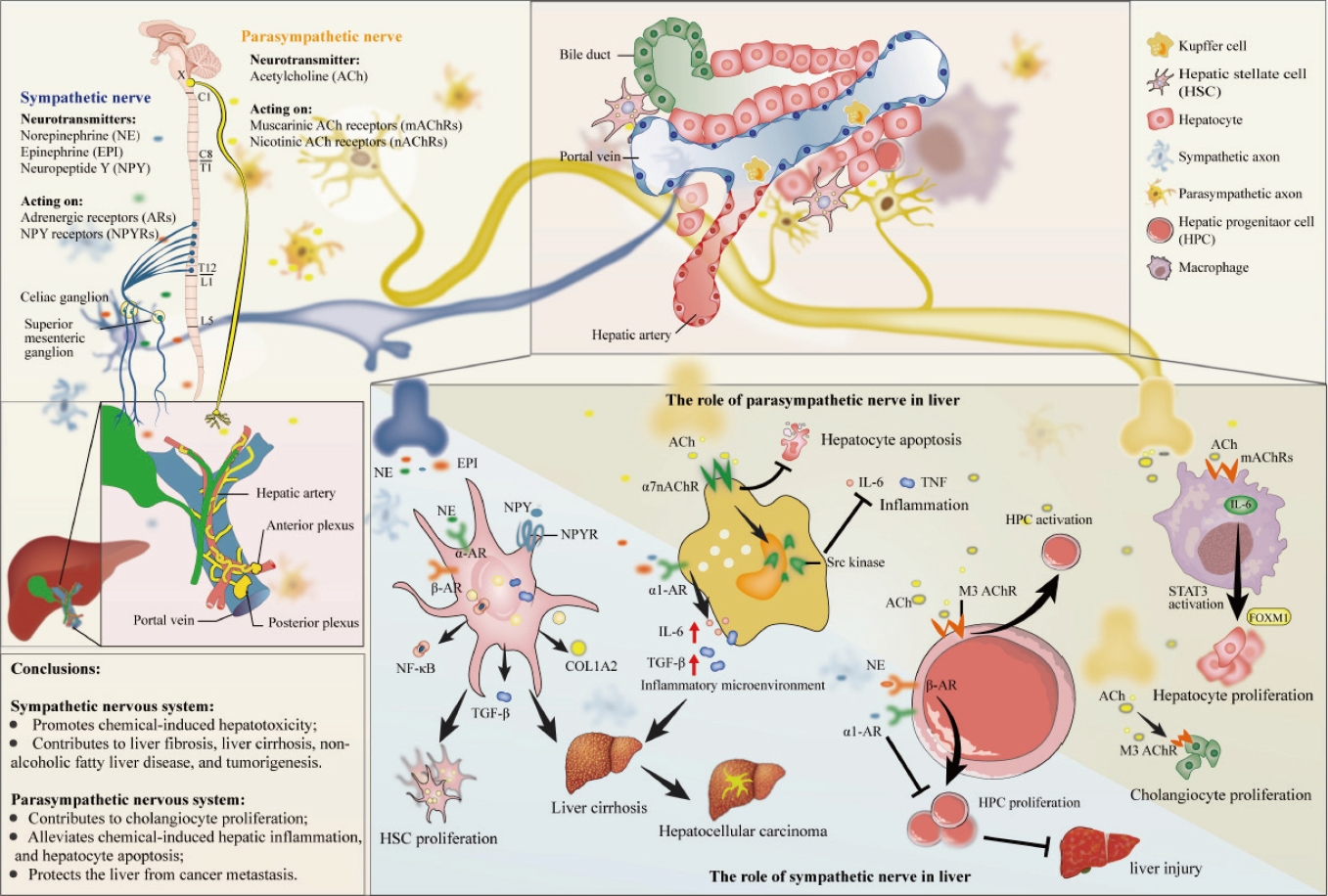
- The role of the hepatic autonomic nervous system
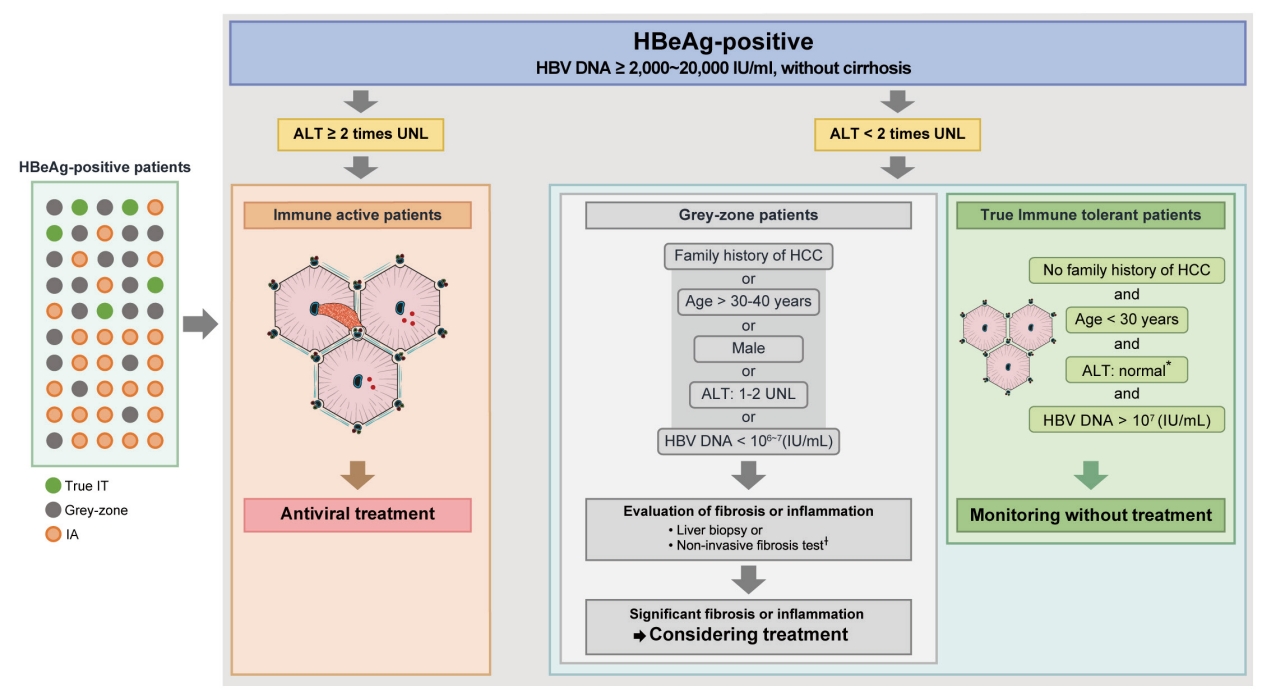
- HBeAg-positive grey-zone patients: Treatment beyond guideline recommendations?
- Systemic therapy in advanced hepatocellular carcinoma

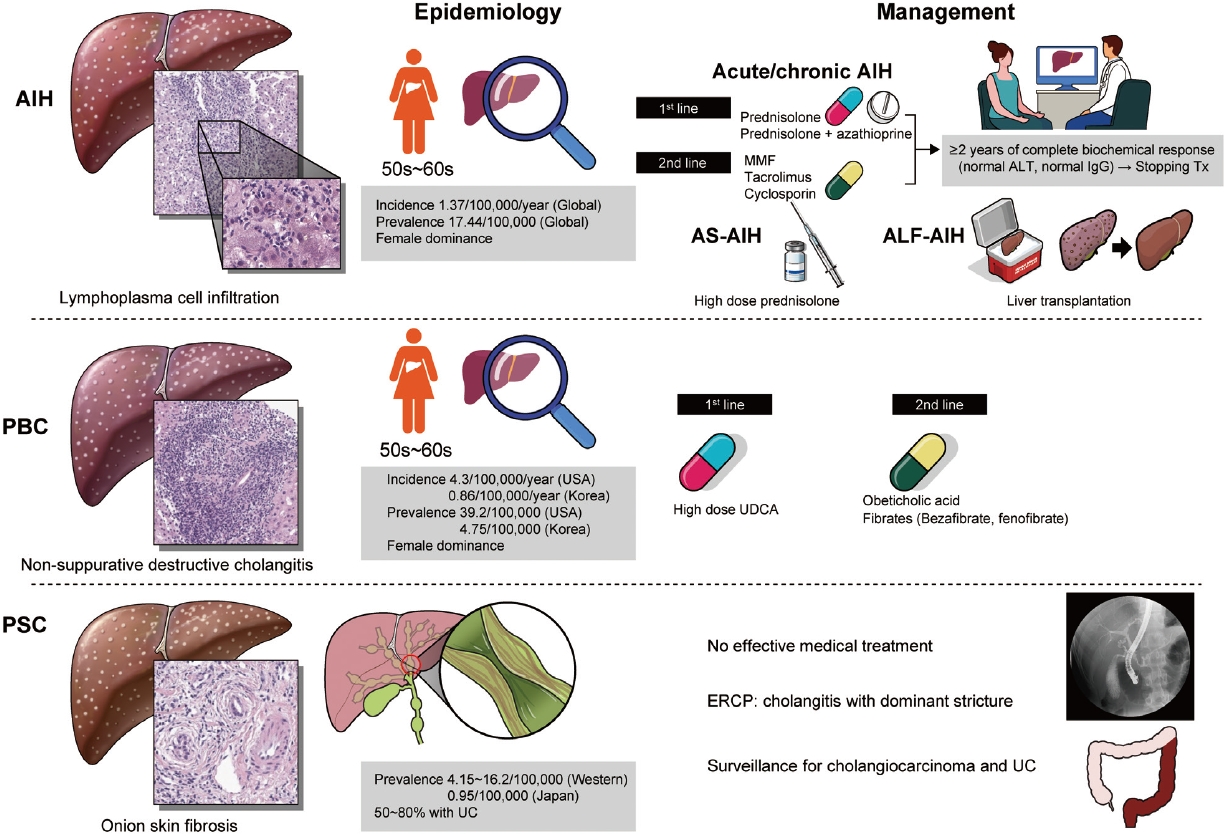
- Epidemiology and updated management for autoimmune liver disease
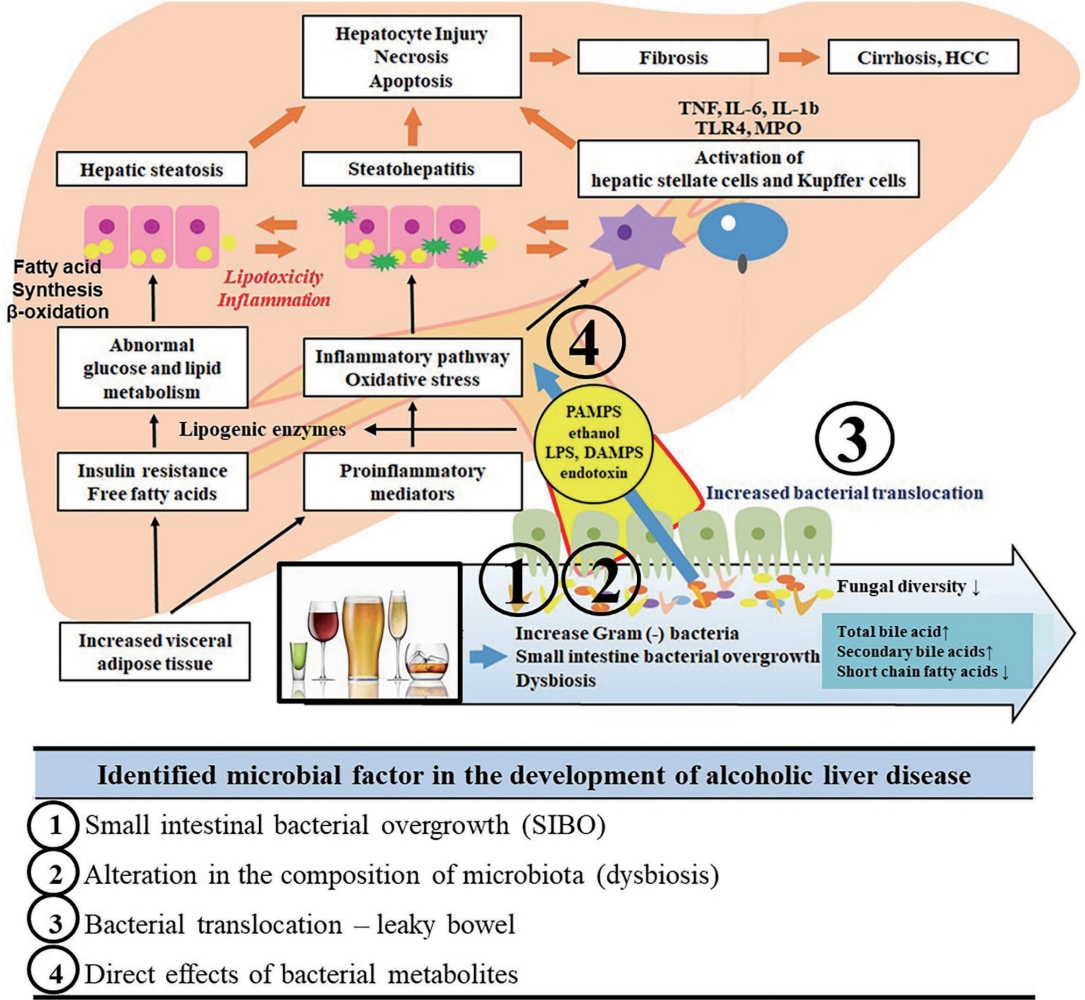
- Microbiome and metabolomics in alcoholic liver disease
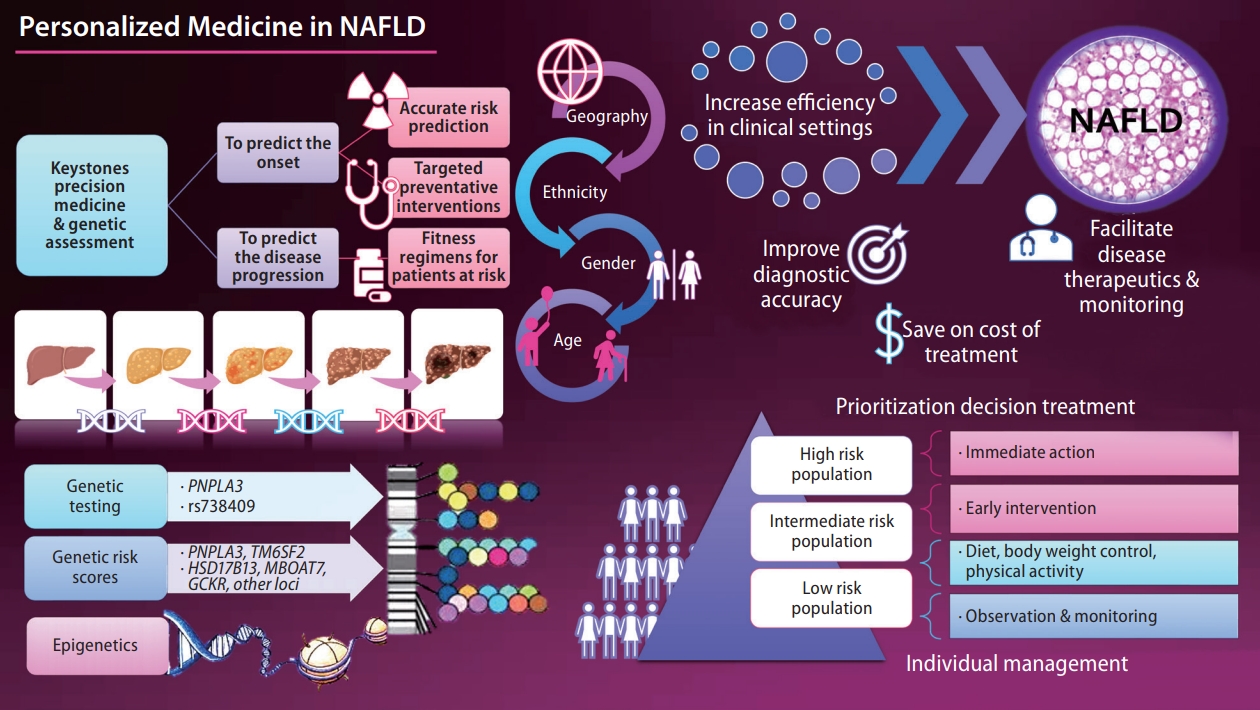
- Personalized medicine in nonalcoholic fatty liver disease
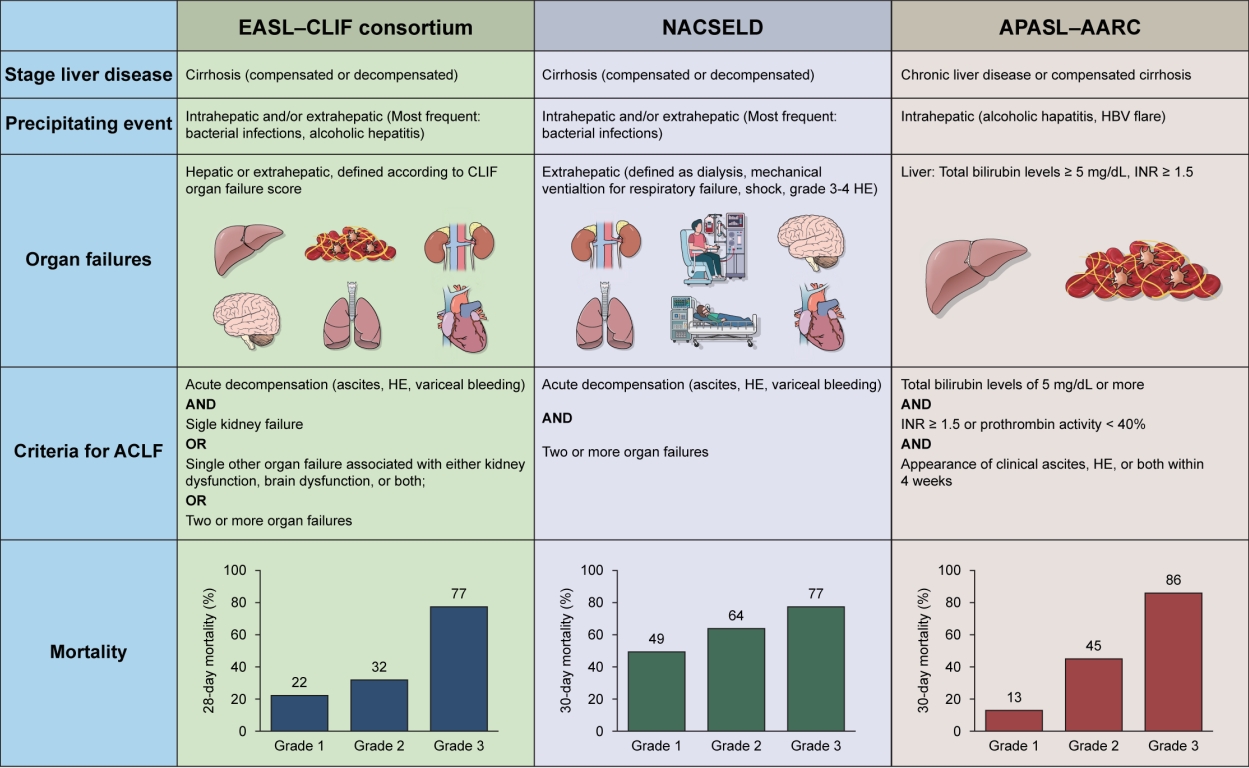
- Acute on chronic liver failure in cirrhosis
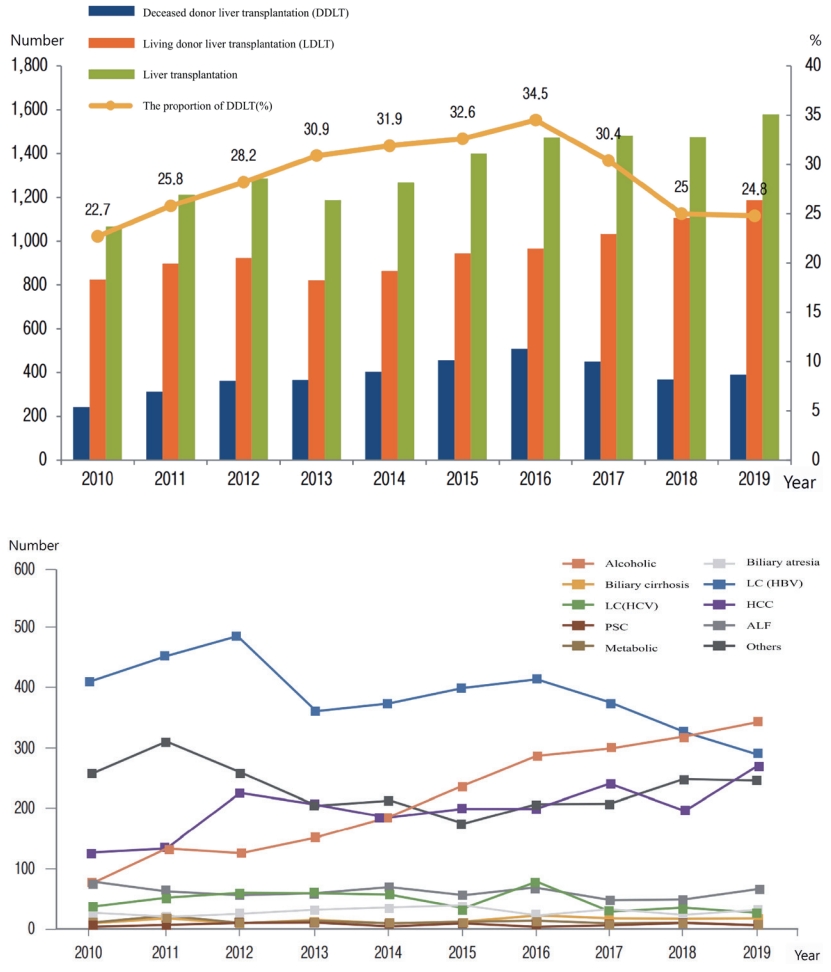
- Current status and outcome of liver transplantation in South Korea
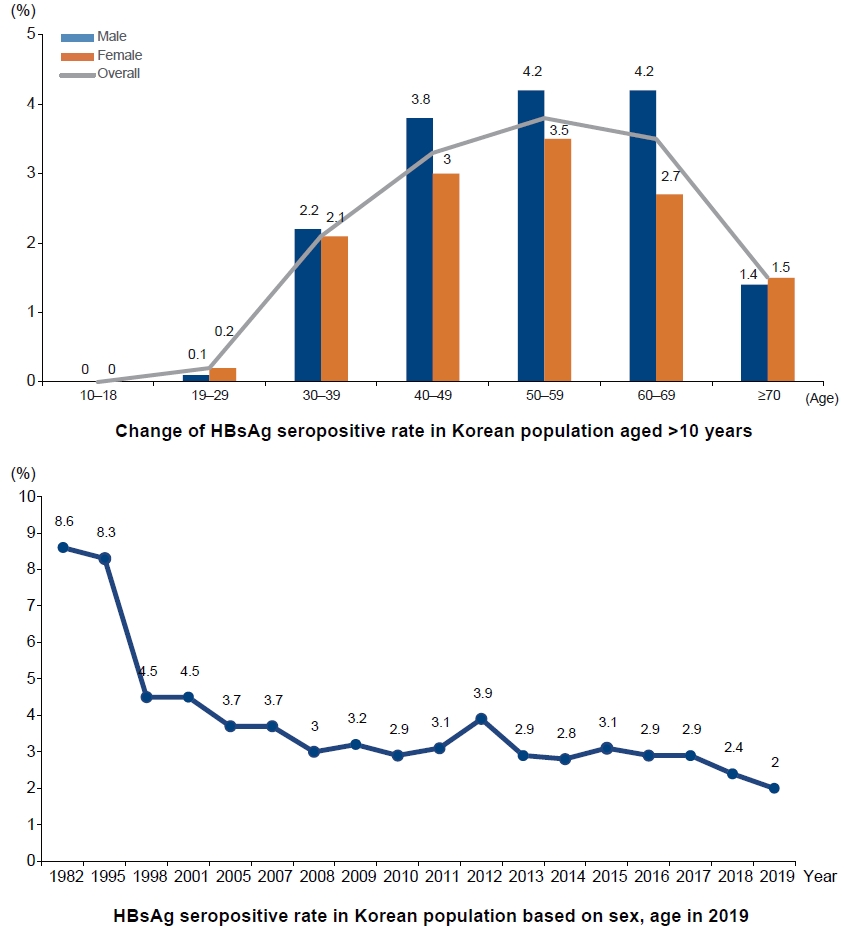
- History and future of hepatitis B virus control in South Korea
- Hepatocellular carcinoma statistics in South Korea


 Impact Factor14.0
Impact Factor14.0First decision
09 Days
Publication after acceptance
03 Days
*Last 12 months
- MOST VIEWED (Last 6 months)
- MOST CITED (2022-2024)







