7. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2095-2128.

11. Korean Central Cancer Registry. Annual report of Korean central cancer registry (2019). Goyang: Korea Central Cancer Registry; 2021.
12. Chon YE, Lee HA, Yoon JS, Park JY, Kim BH, Lee IJ, et al. Hepatocellular carcinoma in Korea between 2012 and 2014: an analysis of data from the Korean nationwide cancer registry. J Liver Cancer 2020;20:135-147.


14. Korean Central Cancer Registry. Annual report of Korean central cancer registry (2015). Goyang: Korea Central Cancer Registry; 2017.
16. Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst 2009;101:1348-1155.


17. World Health Organization (WHO). Hepatitis B vaccines: WHO position paper. Geneva: WHO; 2017.
18. European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-185.


20. Park H, Shin SK, Joo I, Song DS, Jang JW, Park JW, et al. Systematic review with meta-analysis: low-level alcohol consumption and the risk of liver cancer. Gut Liver 2020;14:792-807.


21. Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol 2005;42:218-224.


22. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004;126:460-468.


24. Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology 2013;144:323-332.


27. Kim G, Jang SY, Nam CM, Kang ES. Statin use and the risk of hepatocellular carcinoma in patients at high risk: a nationwide nested case-control study. J Hepatol 2018;68:476-484.


30. Wong YJ, Qiu TY, Ng GK, Zheng Q, Teo EK. Efficacy and safety of statin for hepatocellular carcinoma prevention among chronic liver disease patients: a systematic review and meta-analysis. J Clin Gastroenterol 2021;55:615-623.

32. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 2013;62:606-615.


39. Wang S, Yu Y, Ryan PM, Dang M, Clark C, Kontogiannis V, et al. Association of aspirin therapy with risk of hepatocellular carcinoma: a systematic review and dose-response analysis of cohort studies with 2.5 million participants. Pharmacol Res 2020;151:104585.


41. Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol 2013;11:1413-1421.e1.


42. Inoue M, Yoshimi I, Sobue T, Tsugane S. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst 2005;97:293-300.


43. Gelatti U, Covolo L, Franceschini M, Pirali F, Tagger A, Ribero ML, et al. Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: a case-control study. J Hepatol 2005;42:528-534.


47. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521-1531.


49. Kim WR, Loomba R, Berg T, Aguilar Schall RE, Yee LJ, Dinh PV, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer 2015;121:3631-3638.


50. Colombo M, Iavarone M. Role of antiviral treatment for HCC prevention. Best Pract Res Clin Gastroenterol 2014;28:771-781.


52. Kim SU, Seo YS, Lee HA, Kim MN, Lee YR, Lee HW, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. J Hepatol 2019;71:456-464.


56. Tseng CH, Hsu YC, Chen TH, Ji F, Chen IS, Tsai YN, et al. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:1039-1052.


61. Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol 1997;65:284-297.


62. Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology 2014;147:152-161.


65. Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol 2010;8:280-288 288.e1.


66. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158:329-337.


67. Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 2019;393:1453-1464.


68. Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology 2018;155:411-421.e4.


69. Mendizabal M, Pi├▒ero F, Ridruejo E, Herz Wolff F, Anders M, Reggiardo V, et al. Disease progression in patients with hepatitis C virus infection treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol 2020;18:2554-2563.e3.


70. Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67:1204-1212.


71. Delgado Mart├Łnez C, G├│mez-Rubio M, G├│mez-Dom├Łnguez C. Is hepatitis C direct-acting antiviral therapy a risk factor for the development and recurrence of hepatocellular carcinoma? Narrative literature review and clinical practice recommendations. Ann Hepatol 2021;21:100225.


72. Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol 2013;31:3647-3655.


73. Wong JS, Wong GL, Tsoi KK, Wong VW, Cheung SY, Chong CN, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther 2011;33:1104-1112.


78. Lee JH, Kim BK, Park SY, Tak WY, Park JY, Kim DY, et al. The efficacies of entecavir and tenofovir in terms of enhancing prognosis after curative treatment of hepatitis B virus-related hepatocellular carcinoma. Eur J Intern Med 2021;89:48-55.


79. Singal AK, Freeman DH Jr, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther 2010;32:851-858.


80. Reig M, Mari├▒o Z, Perell├│ C, I├▒arrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719-726.


81. Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727-733.


82. Reig M, Boix L, Mari├▒o Z, Torres F, Forns X, Bruix J. Liver cancer emergence associated with antiviral treatment: an immune surveillance failure? Semin Liver Dis 2017;37:109-118.


84. ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol 2016;65:734-740.


85. Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannav├▓ MR, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol 2019;71:265-273.


89. Nishio T, Taura K, Nakamura N, Seo S, Yasuchika K, Kaido T, et al. Impact of statin use on the prognosis of patients with hepatocellular carcinoma undergoing liver resection: a subgroup analysis of patients without chronic hepatitis viral infection. Surgery 2018;163:264-269.


95. Zhao C, Nguyen MH. Hepatocellular carcinoma screening and surveillance: practice guidelines and real-life practice. J Clin Gastroenterol 2016;50:120-133.

96. Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004;126:1005-1014.


97. Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010;53:291-297.


98. Kim HD, Lim YS, Han S, An J, Kim GA, Kim SY, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology 2015;148:1371-1382.


102. Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol 2017;34:153-159.


104. Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class a cirrhosis. Am J Med 1996;101:422-434.


108. Calleja JL, Crespo J, Rinc├│n D, Ruiz-Antor├Īn B, Fernandez I, Perell├│ C, et al. Effectiveness, safety and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a spanish real-world cohort. J Hepatol 2017;66:1138-1148.


110. Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ann Intern Med 2015;162:697-711.


112. Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol 2006;101:513-523.

114. European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-943.


116. Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC). 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver 2015;9:267-317.


117. Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol 2009;15:391-423.


119. Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 2011;54:1987-1997.


122. Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology 1992;16:132-137.


123. Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology 1985;89:259-266.


124. Tanaka H, Iijima H, Nouso K, Aoki N, Iwai T, Takashima T, et al. Cost-effectiveness analysis on the surveillance for hepatocellular carcinoma in liver cirrhosis patients using contrast-enhanced ultrasonography. Hepatol Res 2012;42:376-384.


125. Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther 2013;38:303-312.


126. Gupta P, Soundararajan R, Patel A, Kumar-M P, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: a systematic review and meta-analysis. J Hepatol 2021;75:108-119.


127. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.


132. Lewis S, Roayaie S, Ward SC, Shyknevsky I, Jibara G, Taouli B. Hepatocellular carcinoma in chronic hepatitis C in the absence of advanced fibrosis or cirrhosis. AJR Am J Roentgenol 2013;200:W610-W616.


133. Kim SE, Lee HC, Shim JH, Park HJ, Kim KM, Kim PN, et al. Noninvasive diagnostic criteria for hepatocellular carcinoma in hepatic masses >2 cm in a hepatitis B virus-endemic area. Liver Int 2011;31:1468-1476.


134. Van Wettere M, Purcell Y, Bruno O, Payanc├® A, Plessier A, Rautou PE, et al. Low specificity of washout to diagnose hepatocellular carcinoma in nodules showing arterial hyperenhancement in patients with budd-chiari syndrome. J Hepatol 2019;70:1123-1132.


139. Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010;59:638-644.


140. Khalili K, Kim TK, Jang HJ, Haider MA, Khan L, Guindi M, et al. Optimization of imaging diagnosis of 1-2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol 2011;54:723-728.


141. Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, et al. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol 2018;68:485-492.


143. Yoon SH, Lee JM, So YH, Hong SH, Kim SJ, Han JK, et al. Multiphasic MDCT enhancement pattern of hepatocellular carcinoma smaller than 3 cm in diameter: tumor size and cellular differentiation. AJR Am J Roentgenol 2009;193:W482-W489.


144. Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology 2005;42:27-34.


145. Kierans AS, Kang SK, Rosenkrantz AB. The diagnostic performance of dynamic contrast-enhanced MR imaging for detection of small hepatocellular carcinoma measuring up to 2 cm: a meta-analysis. Radiology 2016;278:82-94.


146. Choi SH, Byun JH, Lim YS, Yu E, Lee SJ, Kim SY, et al. Diagnostic criteria for hepatocellular carcinoma Ōēż3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol 2016;64:1099-1107.


148. Ahn SJ, Choi JY, Kim KA, Kim MJ, Baek SE, Kim JH, et al. Focal eosinophilic infiltration of the liver: gadoxetic acid-enhanced magnetic resonance imaging and diffusion-weighted imaging. J Comput Assist Tomogr 2011;35:81-85.

149. Kim DH, Choi SH, Byun JH, Kang JH, Lim YS, Lee SJ, et al. Arterial subtraction images of gadoxetate-enhanced MRI improve diagnosis of early-stage hepatocellular carcinoma. J Hepatol 2019;71:534-542.


150. Choi SH, Kim SY, Lee SS, Shim JH, Byun JH, Baek S, et al. Subtraction images of gadoxetic acid-enhanced MRI: effect on the diagnostic performance for focal hepatic lesions in patients at risk for hepatocellular carcinoma. AJR Am J Roentgenol 2017;209:584-591.


153. Min JH, Kim JM, Kim YK, Cha DI, Kang TW, Kim H, et al. Magnetic resonance imaging with extracellular contrast detects hepatocellular carcinoma with greater accuracy than with gadoxetic acid or computed tomography. Clin Gastroenterol Hepatol 2020;18:2091-2100.e7.


154. Reynolds AR, Furlan A, Fetzer DT, Sasatomi E, Borhani AA, Heller MT, et al. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics 2015;35:371-386.


159. Kang HJ, Lee JM, Yoon JH, Lee K, Kim H, Han JK. Contrast-enhanced US with sulfur hexafluoride and perfluorobutane for the diagnosis of hepatocellular carcinoma in individuals with high risk. Radiology 2020;297:108-116.


161. Cerny M, Chernyak V, Olivi├® D, Billiard JS, Murphy-Lavall├®e J, Kielar AZ, et al. LI-RADS version 2018 ancillary features at MRI. Radiographics 2018;38:1973-2001.


162. van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. Accuracy of the liver imaging reporting and data system in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy-a systematic review. Gastroenterology 2019;156:976-986.


163. Tanabe M, Kanki A, Wolfson T, Costa EA, Mamidipalli A, Ferreira MP, et al. Imaging outcomes of liver imaging reporting and data system version 2014 category 2, 3, and 4 observations detected at CT and MR imaging. Radiology 2016;281:129-139.


164. Kim YY, Choi JY, Kim SU, Lee M, Park MS, Chung YE, et al. MRI ancillary features for LI-RADS category 3 and 4 observations: improved categorization to indicate the risk of hepatic malignancy. AJR Am J Roentgenol 2020;215:1354-1362.


165. Vernuccio F, Cannella R, Choudhury KR, Meyer M, Furlan A, Marin D. Hepatobiliary phase hypointensity predicts progression to hepatocellular carcinoma for intermediate-high risk observations, but not time to progression. Eur J Radiol 2020;128:109018.


166. Cho HJ, Kim B, Lee JD, Kang DR, Kim JK, Lee JH, et al. Development of risk prediction model for hepatocellular carcinoma progression of indeterminate nodules in hepatitis B virus-related cirrhotic liver. Am J Gastroenterol 2017;112:460-470.


169. Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology 2012;264:761-770.


171. Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW, Han JK, et al. Small (Ōēż1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology 2014;271:748-760.


173. Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology 2015;275:97-109.


175. Roskams T, Kojiro M. Pathology of early hepatocellular carcinoma: conventional and molecular diagnosis. Semin Liver Dis 2010;30:17-25.


176. Forner A, Vilana R, Ayuso C, Bianchi L, Sol├® M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104.


177. Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev 2007;33:437-447.

178. Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut 2008;57:1592-1596.


179. Tremosini S, Forner A, Boix L, Vilana R, Bianchi L, Reig M, et al. Prospective validation of an immunohistochemical panel (glypican 3, heat shock protein 70 and glutamine synthetase) in liver biopsies for diagnosis of very early hepatocellular carcinoma. Gut 2012;61:1481-1487.


180. WHO Classification of Tumours Editorial Board. WHO classification of tumors: digestive system tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2019.
182. Kim H, Jang M, Park YN. Histopathological variants of hepatocellular carcinomas: an update according to the 5th edition of the WHO classification of digestive system tumors. J Liver Cancer 2020;20:17-24.


183. Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol 2019;71:616-630.


185. Chen VL, Sharma P. Role of biomarkers and biopsy in hepatocellular carcinoma. Clin Liver Dis 2020;24:577-590.


187. Wong RJ, Ahmed A, Gish RG. Elevated alpha-fetoprotein: differential diagnosis - hepatocellular carcinoma and other disorders. Clin Liver Dis 2015;19:309-323.

189. Hwang SH, Hong SB, Han K, Seo N, Choi JY, Lee JH, et al. A new reporting system for diagnosis of hepatocellular carcinoma in chronic hepatitis B with clinical and gadoxetic acid-enhanced MRI features. J Magn Reson Imaging 2022;55:1877-1886.

190. Joo I, Kim SY, Kang TW, Kim YK, Park BJ, Lee YJ, et al. Radiologic-pathologic correlation of hepatobiliary phase hypointense nodules without arterial phase hyperenhancement at gadoxetic acid-enhanced MRI: a multicenter study. Radiology 2020;296:335-345.


192. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947-955.

193. Gilbert ES. Invited commentary: studies of workers exposed to low doses of radiation. Am J Epidemiol 2001;153:319-322 discussion 323-324.


194. Upton AC; National Coluncil on Radiation Protection and Measurements Scientific Committee 1-6. The state of the art in the 1990ŌĆÖs: NCRP report No. 136 on the scientific bases for linearity in the dose-response relationship for ionizing radiation. Health Phys 2003;85:15-22.


196. National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. Washington, D.C.: National Academy of Sciences; 2006.
197. The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP 2007;37:1-332.
198. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology 2011;261:193-198.


199. Takahashi H, Okada M, Hyodo T, Hidaka S, Kagawa Y, Matsuki M, et al. Can low-dose CT with iterative reconstruction reduce both the radiation dose and the amount of iodine contrast medium in a dynamic CT study of the liver? Eur J Radiol 2014;83:684-691.


202. Yoon JH, Chang W, Lee ES, Lee SM, Lee JM. Double low-dose dual-energy liver CT in patients at high-risk of HCC: a prospective, randomized, single-center study. Invest Radiol 2020;55:340-348.


204. DŌĆÖAmico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217-231.


205. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003;37:429-442.


206. Lee HW, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, et al. Cause of mortality for hepatocellular carcinoma patients who were diagnosed within the milan criteria. J Liver Cancer 2016;16:101-107.

210. Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res 2002;24:395-403.


211. Nihon Kangan K. General rules for the clinical and pathological study of primary liver cancer. Tokyo: Kanehara & Co.; 2010.
214. Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-1236.


215. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-1700.e3.


217. Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, Kondo Y, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 2011;117:4475-4483.


218. Benson AB, DŌĆÖAngelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021;19:541-565.

219. Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med 2008;49:1912-1921.


224. Chalaye J, Costentin CE, Luciani A, Amaddeo G, Ganne-Carri├® N, Baranes L, et al. Positron emission tomography/computed tomography with 18F-fluorocholine improve tumor staging and treatment allocation in patients with hepatocellular carcinoma. J Hepatol 2018;69:336-344.


225. Rilling WS, Drooz A. Multidisciplinary management of hepatocellular carcinoma. J Vasc Interv Radiol 2002;13:S259-S263.


226. Colombo M, Raoul JL, Lencioni R, Galle PR, Zucman-Rossi J, Ba├▒ares R, et al. Multidisciplinary strategies to improve treatment outcomes in hepatocellular carcinoma: a European perspective. Eur J Gastroenterol Hepatol 2013;25:639-651.

234. Chan AC, Poon RT, Ng KK, Lo CM, Fan ST, Wong J. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg 2008;247:666-673.


235. Taylor C, Munro AJ, Glynne-Jones R, Griffith C, Trevatt P, Richards M, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ 2010;340:c951.


237. Gish RG, Lencioni R, Di Bisceglie AM, Raoul JL, Mazzaferro V. Role of the multidisciplinary team in the diagnosis and treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol 2012;6:173-185.


240. Capussotti L, Muratore A, Massucco P, Ferrero A, Polastri R, Bouzari H. Major liver resections for hepatocellular carcinoma on cirrhosis: early and long-term outcomes. Liver Transpl 2004;10(2 Suppl 1):S64-S68.


245. Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: An observational study of the HCC east-west study group. Ann Surg 2013;257:929-937.

246. Kim JH, Choi DW, Kim SB. Saftey and long-term outcome following major hepatectomy for epatocellular carcinoma combined with compensated liver cirrhosis. J Korean Surg Soc 2006;70:445-450.
248. Finkelstein SD, Marsh W, Demetris AJ, Swalsky PA, Sasatomi E, Bonham A, et al. Microdissection-based allelotyping discriminates de novo tumor from intrahepatic spread in hepatocellular carcinoma. Hepatology 2003;37:871-879.


249. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200-207.


251. Li SH, Guo ZX, Xiao CZ, Wei W, Shi M, Chen ZY, et al. Risk factors for early and late intrahepatic recurrence in patients with single hepatocellular carcinoma without macrovascular invasion after curative resection. Asian Pac J Cancer Prev 2013;14:4759-4763.


252. Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg 2009;249:799-805.


253. Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229-235.


254. Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol 2009;51:890-897.


255. Zhou L, Rui JA, Wang SB, Chen SG, Qu Q. Prognostic factors of solitary large hepatocellular carcinoma: the importance of differentiation grade. Eur J Surg Oncol 2011;37:521-525.


259. Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38-46.

260. Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg 1999;229:210-215.


261. Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl 2004;10(2 Suppl 1):S46-S52.


262. Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg 1995;130:198-203.


263. Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434-1440.


264. An M, Park J, Shin JA, Choi JI, Kim TH, Kim S, et al. The adverse effect of indirectly diagnosed portal hypertension on the complications and prognosis after hepatic resection of hepatocellular carcinoma. Korean J Hepatol 2006;12:553.

265. Choi GH, Park JY, Hwang HK, Kim DH, Kang CM, Choi JS, et al. Predictive factors for long-term survival in patients with clinically significant portal hypertension following resection of hepatocellular carcinoma. Liver Int 2011;31:485-493.


267. Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg 2009;250:922-928.


269. Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008;134:1908-1916.


271. Cescon M, Colecchia A, Cucchetti A, Peri E, Montrone L, Ercolani G, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg 2012;256:706-712 discussion 712-713.


274. Wong JS, Wong GL, Chan AW, Wong VW, Cheung YS, Chong CN, et al. Liver stiffness measurement by transient elastography as a predictor on posthepatectomy outcomes. Ann Surg 2013;257:922-928.


275. Li C, Zhang JY, Zhang XY, Wen TF, Yan LN. FibroScan predicts ascites after liver resection for hepatitis B virus-related hepatocellular carcinoma: a prospective cohort study. Int J Surg 2015;20:21-25.


277. Rajakannu M, Cherqui D, Ciacio O, Golse N, Pittau G, Allard MA, et al. Liver stiffness measurement by transient elastography predicts late posthepatectomy outcomes in patients undergoing resection for hepatocellular carcinoma. Surgery 2017;162:766-774.


280. Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, et al. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol 2009;192:1675-1681.


281. Ippolito D, Famularo S, Giani A, Orsini EB, Pecorelli A, Pinotti E, et al. Estimating liver function in a large cirrhotic cohort: signal intensity of gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced MRI. Dig Liver Dis 2019;51:1438-1445.


282. Lin CY, Chang WC, Chou CT, Chen RC. Dynamic-contrast-enhanced magnetic resonance imaging of cirrhotic liver parenchyma: a comparison between gadolinium-diethylenetriamine pentaacetic acid and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid. J Chin Med Assoc 2015;78:666-672.


285. Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Radiol 2012;81:2417-2422.


290. Tsujita E, Taketomi A, Kitagawa D, Itoh S, Harimoto N, Gion T, et al. Selective hepatic vascular exclusion for the hepatic resection of HCC. Hepatogastroenterology 2007;54:527-530.

291. Jongerius IM, Mungroop TH, Uz Z, Geerts BF, Immink RV, Rutten MVH, et al. Goal-directed fluid therapy vs. low central venous pressure during major open liver resections (GALILEO): a surgeon- and patient-blinded randomized controlled trial. HPB (Oxford) 2021;23:1578-1585.


294. Cucchetti A, Qiao GL, Cescon M, Li J, Xia Y, Ercolani G, et al. Anatomic versus nonanatomic resection in cirrhotic patients with early hepatocellular carcinoma. Surgery 2014;155:512-521.


297. Kudo A, Tanaka S, Ban D, Matsumura S, Irie T, Nakamura N, et al. Anatomic resection reduces the recurrence of solitary hepatocellular carcinoma Ōēż5 cm without macrovascular invasion. Am J Surg 2014;207:863-869.


300. Huang X, Lu S. A meta-analysis comparing the effect of anatomical resection vs. non-anatomical resection on the long-term outcomes for patients undergoing hepatic resection for hepatocellular carcinoma. HPB (Oxford) 2017;19:843-849.


301. Feng X, Su Y, Zheng S, Xia F, Ma K, Yan J, et al. A double blinded prospective randomized trial comparing the effect of anatomic versus non-anatomic resection on hepatocellular carcinoma recurrence. HPB (Oxford) 2017;19:667-674.


302. Moris D, Tsilimigras DI, Kostakis ID, Ntanasis-Stathopoulos I, Shah KN, Felekouras E, et al. Anatomic versus non-anatomic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Surg Oncol 2018;44:927-938.


303. Sun Z, Li Z, Shi XL, He XW, Chen J, Song JH. Anatomic versus non-anatomic resection of hepatocellular carcinoma with microvascular invasion: a systematic review and meta-analysis. Asian J Surg 2021;44:1143-1150.


305. Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg 2007;245:36-43.


306. Zhong FP, Zhang YJ, Liu Y, Zou SB. Prognostic impact of surgical margin in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2017;96:e8043.


307. Lazzara C, Navarra G, Lazzara S, Barbera A, Saitta C, Raimondo G, et al. Does the margin width influence recurrence rate in liver surgery for hepatocellular carcinoma smaller than 5 cm? Eur Rev Med Pharmacol Sci 2017;21:523-529.

308. Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery 2008;143:469-475.


311. Kim IS, Lim YS, Yoon HK, Sung KB, Jang MK, Choi WB, et al. The effect of preoperative transarterial chemoembolization on the patientŌĆÖs outcome in resectable hepatocellular carcinoma. Korean J Med 2005;69:614-621.
314. Hayashi H, Beppu T, Okabe H, Kuroki H, Nakagawa S, Imai K, et al. Functional assessment versus conventional volumetric assessment in the prediction of operative outcomes after major hepatectomy. Surgery 2015;157:20-26.


315. Nishio T, Taura K, Koyama Y, Tanabe K, Yamamoto G, Okuda Y, et al. Prediction of posthepatectomy liver failure based on liver stiffness measurement in patients with hepatocellular carcinoma. Surgery 2016;159:399-408.


316. Beppu T, Okabe H, Okuda K, Eguchi S, Kitahara K, Taniai N, et al. Portal vein embolization followed by right-side hemihepatectomy for hepatocellular carcinoma patients: a Japanese multi-institutional study. J Am Coll Surg 2016;222:1138-1148.e2.


317. Pandanaboyana S, Bell R, Hidalgo E, Toogood G, Prasad KR, Bartlett A, et al. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery 2015;157:690-698.


318. Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg 2015;262:780-785 discussion 785-786.

319. Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, et al. ALPPS versus portal vein embolization for hepatitis-related hepatocellular carcinoma: a changing paradigm in modulation of future liver remnant before major hepatectomy. Ann Surg 2021;273:957-965.

320. Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg 2001;193:109-111.


322. Han HS, Shehta A, Ahn S, Yoon YS, Cho JY, Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol 2015;63:643-650.


324. Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg 2013;257:506-511.

325. Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee SY, et al. Consensus guidelines for the use of fluorescence imaging in hepatobiliary surgery. Ann Surg 2021;274:97-106.


326. Benedetti Cacciaguerra A, G├Črgec CSC, Lanari J, Cipriani F, Russolillo N, Mocchegiani F, et al. Outcome of major hepatectomy in cirrhotic patients; does surgical approach matter? A propensity score matched analysis. J Hepatobiliary Pancreat Sci 2021 Dec 2;oi:
10.1002/jhbp.1087.
327. Yoon YI, Kim KH, Kang SH, Kim WJ, Shin MH, Lee SK, et al. Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score matched analysis. Ann Surg 2017;265:856-863.

328. Berardi G, Morise Z, Sposito C, Igarashi K, Panetta V, Simonelli I, et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J Hepatol 2020;72:75-84.


329. Troisi RI, Berardi G, Morise Z, Cipriani F, Ariizumi S, Sposito C, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg 2021;108:196-204.

331. Pesi B, Bencini L, Moraldi L, Tofani F, Batignani G, Bechi P, et al. Robotic versus open liver resection in hepatocarcinoma: surgical and oncological outcomes. Surg Laparosc Endosc Percutan Tech 2021;31:468-474.


337. Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, et al. Liver resection for multiple hepatocellular carcinomas: a Japanese nationwide survey. Ann Surg 2020;272:145-154.

338. Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell 2003;113:495-506.


339. Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854-862 discussion 862-864.


341. Aoki T, Kokudo N, Matsuyama Y, Izumi N, Ichida T, Kudo M, et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg 2014;259:532-542.

342. Li J, Huang L, Liu CF, Cao J, Yan JJ, Xu F, et al. Risk factors and surgical outcomes for spontaneous rupture of BCLC stages A and B hepatocellular carcinoma: a case-control study. World J Gastroenterol 2014;20:9121-9127.


346. Wu JJ, Zhu P, Zhang ZG, Zhang BX, Shu C, MbaŌĆÖnbo-Koumpa AA, et al. Spontaneous rupture of hepatocellular carcinoma: optimal timing of partial hepatectomy. Eur J Surg Oncol 2019;45:1887-1894.


349. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938-943.


352. Zhang ZY, Dong KS, Zhang EL, Zhang LW, Chen XP, Dong HH. Resection might be a meaningful choice for hepatocellular carcinoma with portal vein thrombosis: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e18362.


356. Kim DS, Kim BW, Hatano E, Hwang S, Hasegawa K, Kudo A, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korea-Japan multicenter study. Ann Surg 2020;271:913-921.


360. Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncology 2020;6:255-263.

361. Wang HL, Mo DC, Zhong JH, Ma L, Wu FX, Xiang BD, et al. Systematic review of treatment strategy for recurrent hepatocellular carcinoma: salvage liver transplantation or curative locoregional therapy. Medicine (Baltimore) 2019;98:e14498.


362. Okada S, Shimada K, Yamamoto J, Takayama T, Kosuge T, Yamasaki S, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology 1994;106:1618-1624.


363. Shirabe K, Kanematsu T, Matsumata T, Adachi E, Akazawa K, Sugimachi K. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology 1991;14:802-805.


364. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999;229:216-222.


365. Adachi E, Maeda T, Matsumata T, Shirabe K, Kinukawa N, Sugimachi K, et al. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology 1995;108:768-775.


366. Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg 2021;273:564-571.


368. Agopian VG, Harlander-Locke MP, Ruiz RM, Klintmalm GB, Senguttuvan S, Florman SS, et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within milan criteria undergoing liver transplantation: analysis of 3601 patients from the US multicenter HCC transplant consortium. Ann Surg 2017;266:525-535.

369. Kim BK, Park JY, Kim DY, Kim JK, Kim KS, Choi JS, et al. Persistent hepatitis B viral replication affects recurrence of hepatocellular carcinoma after curative resection. Liver Int 2008;28:393-401.


370. Ohkubo K, Kato Y, Ichikawa T, Kajiya Y, Takeda Y, Higashi S, et al. Viral load is a significant prognostic factor for hepatitis B virus-associated hepatocellular carcinoma. Cancer 2002;94:2663-2668.


371. Hung IF, Poon RT, Lai CL, Fung J, Fan ST, Yuen MF. Recurrence of hepatitis B-related hepatocellular carcinoma is associated with high viral load at the time of resection. Am J Gastroenterol 2008;103:1663-1673.


373. Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma - a systematic review. Surg Oncol 2013;22:e23-e30.


376. Poon RT, Fan ST, OŌĆÖSuilleabhain CB, Wong J. Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J Am Coll Surg 2002;195:311-318.

378. Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology 2012;262:689-700.


381. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-699.


384. Germani G, Gurusamy K, Garcovich M, Toso C, Fede G, Hemming A, et al. Which matters most: number of tumors, size of the largest tumor, or total tumor volume? Liver Transpl 2011;17 Suppl 2:S58-S66.


385. Sugimachi K, Shirabe K, Taketomi A, Soejima Y, Iguchi T, Takeishi K, et al. Prognostic significance of preoperative imaging in recipients of living donor liver transplantation for hepatocellular carcinoma. Transplantation 2011;91:570-574.


389. Lim C, Salloum C, Chalaye J, Lahat E, Costentin CE, Osseis M, et al. 18F-FDG PET/CT predicts microvascular invasion and early recurrence after liver resection for hepatocellular carcinoma: a prospective observational study. HPB (Oxford) 2019;21:739-747.

390. Alcorn JB; United Network for Organ Sharing. Changes to OPTN by laws and policies from actions at November board of directors meeting. 2016.
391. Heimbach JK. Evolution of liver transplant selection criteria and U.S. allocation policy for patients with hepatocellular carcinoma. Semin Liver Dis 2020;40:358-364.


392. Korean Organ Donation Agency. Korea Network for Organ Sharing (KONOS) Registry. 2020;2020:15-17.
393. Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant 2006;6:1416-1421.


394. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-1403.


396. Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg 2004;240:900-909.


398. Mehta N, Heimbach J, Lee D, Dodge JL, Harnois D, Burns J, et al. Wait time of less than 6 and greater than 18 months predicts hepatocellular carcinoma recurrence after liver transplantation: proposing a wait time ŌĆ£sweet spotŌĆØ. Transplantation 2017;101:2071-2078.


401. Lesurtel M, M├╝llhaupt B, Pestalozzi BC, Pfammatter T, Clavien PA. Transarterial chemoembolization as a bridge to liver transplantation for hepatocellular carcinoma: an evidence-based analysis. Am J Transplant 2006;6:2644-2650.


405. Porrett PM, Peterman H, Rosen M, Sonnad S, Soulen M, Markmann JF, et al. Lack of benefit of pre-transplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl 2006;12:665-673.


406. Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg 2008;248:617-625.


408. Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl 2002;8:873-883.


410. Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 2017;67:92-99.


411. De Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, et al. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant 2009;9:1158-1168.


413. Yao FY, Hirose R, LaBerge JM, Davern TJ 3rd, Bass NM, Kerlan RK Jr, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl 2005;11:1505-1514.


414. Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-1928.


415. Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: a systematic review and pooled analysis. Liver Transpl 2015;21:1142-1152.


417. Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol 2020;21:947-956.


418. Chapman WC, Garcia-Aroz S, Vachharajani N, Fowler K, Saad N, Lin Y, et al. Liver transplantation for advanced hepatocellular carcinoma after downstaging without up-front stage restrictions. J Am Coll Surg 2017;224:610-621.


419. Kim JH, Sinn DH, Gwak GY, Choi GS, Kim JM, Kwon CHD, et al. Factors determining long-term outcomes of hepatocellular carcinoma within the Milan criteria: liver transplantation versus locoregional therapy: a retrospective cohort study. Medicine (Baltimore) 2016;95:e4735.


420. Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant 2008;8:2547-2557.


422. Halazun KJ, Sapisochin G, von Ahrens D, Agopian VG, Tabrizian P. Predictors of outcome after liver transplantation for hepatocellular carcinoma (HCC) beyond Milan criteria. Int J Surg 2020;82S:61-69.


423. DiNorcia J, Florman SS, Haydel B, Tabrizian P, Ruiz RM, Klintmalm GB, et al. Pathologic response to pretransplant locoregional therapy is predictive of patient outcome after liver transplantation for hepatocellular carcinoma: analysis from the US multicenter HCC transplant consortium. Ann Surg 2020;271:616-624.

428. Korean Organ Donation Agency. Korea Network for Organ Sharing (KONOS) Registry. Cardiovasc Intervent Radiol 2017;2017:15-22.
429. KONOS. KONOS annual report. Seoul: KONOS; 2019.
430. Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant 2013;27:140-147.


431. Liang W, Wu L, Ling X, Schroder PM, Ju W, Wang D, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl 2012;18:1226-1236.


433. Azoulay D, Audureau E, Bhangui P, Belghiti J, Boillot O, Andreani P, et al. Living or brain-dead donor liver transplantation for hepatocellular carcinoma: a multicenter, western, intent-to-treat cohort study. Ann Surg 2017;266:1035-1044.

434. Bhangui P, Vibert E, Majno P, Salloum C, Andreani P, Zocrato J, et al. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology 2011;53:1570-1579.


436. Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology 2004;127(5 Suppl 1):S277-S282.


437. Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: a life-expectancy and cost-effectiveness perspective. Hepatology 2001;33:1073-1079.


441. Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-945.


443. Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl 2007;13:1637-1644.


444. Taketomi A, Sanefuji K, Soejima Y, Yoshizumi T, Uhciyama H, Ikegami T, et al. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation 2009;87:531-537.


445. Todo S, Furukawa H, Tada M; Japanese Liver Transplantation Study Group. Extending indication: role of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2007;13(11 Suppl 2):S48-S54.


446. Lee JH, Cho Y, Kim HY, Cho EJ, Lee DH, Yu SJ, et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the milan criteria. Ann Surg 2016;263:842-850.


450. Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including ╬▒-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-994.e3 quiz e14-e15.


451. Notarpaolo A, Layese R, Magistri P, Gambato M, Colledan M, Magini G, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol 2017;66:552-559.


453. Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 2013;58:89-97.


454. Shin M, Song S, Kim JM, Kwon CH, Kim SJ, Lee SK, et al. Donor morbidity including biliary complications in living-donor liver transplantation: single-center analysis of 827 cases. Transplantation 2012;93:942-948.

456. Hwang S, Lee SG, Lee YJ, Sung KB, Park KM, Kim KH, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl 2006;12:920-927.


457. Yi NJ, Suh KS, Cho JY, Lee HW, Cho EH, Yang SH, et al. Three-quarters of right liver donors experienced postoperative complications. Liver Transpl 2007;13:797-806.


460. Yang JD, Lee KW, Kim JM, Kim MS, Lee JG, Kang KJ, et al. A comparative study of postoperative outcomes between minimally invasive living donor hepatectomy and open living donor hepatectomy: the Korean organ transplantation registry. Surgery 2021;170:271-276.


461. Rho SY, Lee JG, Joo DJ, Kim MS, Kim SI, Han DH, et al. Outcomes of robotic living donor right hepatectomy from 52 consecutive cases: comparison with open and laparoscopy-assisted donor hepatectomy. Ann Surg 2022;275:e433-e442.


462. Chan SC, Chan AC, Sharr WW, Chok KS, Cheung TT, Fan ST, et al. Perpetuating proficiency in donor right hepatectomy for living donor liver transplantation. Asian J Surg 2014;37:65-72.


464. Siegler M, Simmerling MC, Siegler JH, Cronin DC 2nd. Recipient deaths during donor surgery: a new ethical problem in living donor liver transplantation (LDLT). Liver Transpl 2006;12:358-360.


466. Choi JY, Kim JH, Kim JM, Kim HJ, Ahn HS, Joh JW. Outcomes of living liver donors are worse than those of matched healthy controls. J Hepatol 2022;76:628-638.


467. Schwartz M, Roayaie S, Llovet J. How should patients with hepatocellular carcinoma recurrence after liver transplantation be treated? J Hepatol 2005;43:584-589.


472. Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl 2004;10:534-540.


473. Hollebecque A, Decaens T, Boleslawski E, Mathurin P, Duvoux C, Pruvot FR, et al. Natural history and therapeutic management of recurrent hepatocellular carcinoma after liver transplantation. Gastroenterol Clin Biol 2009;33:361-369.


474. Kim YS, Lim HK, Rhim H, Lee WJ, Joh JW, Park CK. Recurrence of hepatocellular carcinoma after liver transplantation: patterns and prognostic factors based on clinical and radiologic features. AJR Am J Roentgenol 2007;189:352-358.


475. Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg 2017;266:118-125.

476. Roh YN, Kwon CHD, Song S, Shin M, Kim JM, Kim S, et al. The prognosis and treatment outcomes of patients with recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant 2014;28:141-148.


479. Huang J, Yan L, Wu H, Yang J, Liao M, Zeng Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res 2016;200:122-130.


480. Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, et al. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol 2010;21:333-338.


481. Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant 2014;19:309-316.


482. Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, et al. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol 2013;59:59-66.


483. Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl Int 2012;25:1158-1164.


484. Bhoori S, Toffanin S, Sposito C, Germini A, Pellegrinelli A, Lampis A, et al. Personalized molecular targeted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol 2010;52:771-775.


485. Invernizzi F, Iavarone M, Zavaglia C, Mazza S, Maggi U, Cesarini L, et al. Experience with early sorafenib treatment with mTOR inhibitors in hepatocellular carcinoma recurring after liver transplantation. Transplantation 2020;104:568-574.


486. Waghray A, Balci B, El-Gazzaz G, Kim R, Pelley R, Narayanan Menon KV, et al. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant 2013;27:555-561.


491. Biondani P, De Martin E, Samuel D. Safety of an anti-PD-1 immune checkpoint inhibitor in a liver transplant recipient. Ann Oncol 2018;29:286-287.


492. Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, et al. Society of interventional radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part II: recommendations: endorsed by the Canadian association for interventional radiology and the cardiovascular and interventional radiological society of Europe. J Vasc Interv Radiol 2019;30:1168-1184.e1.

494. Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005;234:961-967.


495. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology 2004;127:1714-1723.


496. Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122-130.


497. Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228:235-240.


499. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008;47:82-89.


503. Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology 2015;276:274-285.


504. Lee S, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol 2018;69:70-78.


506. Lee DH, Lee JM, Lee JY, Kim SH, Kim JH, Yoon JH, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MRI: risk of HCC recurrence after radiofrequency ablation. J Hepatol 2015;62:1122-1130.


509. Lee DH, Lee MW, Kim PN, Lee YJ, Park HS, Lee JM. Outcome of no-touch radiofrequency ablation for small hepatocellular carcinoma: a multicenter clinical trial. Radiology 2021;301:229-236.


510. Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev 2017;3:CD011650.


513. Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794-802.


514. Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-912.


517. Yang HJ, Lee JH, Lee DH, Yu SJ, Kim YJ, Yoon JH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology 2014;271:909-918.


518. Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection--propensity score analyses of long-term outcomes. Radiology 2015;275:908-919.


520. Qi X, Tang Y, An D, Bai M, Shi X, Wang J, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: a meta-analysis of randomized controlled trials. J Clin Gastroenterol 2014;48:450-457.

521. Imai K, Yamashita YI, Yusa T, Nakao Y, Itoyama R, Nakagawa S, et al. Microvascular invasion in small-sized hepatocellular carcinoma: significance for outcomes following hepatectomy and radiofrequency ablation. Anticancer Res 2018;38:1053-1060.

523. Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology 2009;252:905-913.


524. Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010;116:5452-5460.


525. Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-432.


526. Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol 2013;25:187-194.

529. de Ba├©re T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol 2003;181:695-700.


530. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics 2003;23:123-134 discussion 134-136.


532. Ahn SJ, Lee JM, Lee DH, Lee SM, Yoon JH, Kim YJ, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol 2017;66:347-354.


535. Ishii H, Okada S, Nose H, Okusaka T, Yoshimori M, Takayama T, et al. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer 1996;77:1792-1796.


536. Vilana R, Bruix J, Bru C, Ayuso C, Sol├® M, Rod├®s J. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology 1992;16:353-357.


537. Livraghi T, Bolondi L, Lazzaroni S, Marin G, Morabito A, Rapaccini GL, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer 1992;69:925-929.


538. Khan KN, Yatsuhashi H, Yamasaki K, Yamasaki M, Inoue O, Koga M, et al. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol 2000;32:269-278.


539. Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol 2008;43:727-735.


540. Giorgio A, Di Sarno A, De Stefano G, Scognamiglio U, Farella N, Mariniello A, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer research 2011;31:2291-2295.

541. Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009;49:453-459.


545. Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, et al. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol 2005;43:458-464.


547. Lencioni R, Llovet JM. Percutaneous ethanol injection for hepatocellular carcinoma: alive or dead? J Hepatol 2005;43:377-380.


550. Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aub├® C, Digklia A, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol 2018;3:317-325.


551. Chong CCN, Lee KF, Cheung SYS, Chu CCM, Fong AKW, Wong J, et al. Prospective double-blinded randomized controlled trial of microwave versus radiofrequency ablation for hepatocellular carcinoma (McRFA trial). HPB (Oxford) 2020;22:1121-1127.


555. Zaitoun MMA, Elsayed SB, Zaitoun NA, Soliman RK, Elmokadem AH, Farag AA, et al. Combined therapy with conventional trans-arterial chemoembolization (cTACE) and microwave ablation (MWA) for hepatocellular carcinoma >3-<5 cm. Int J Hyperthermia 2021;38:248-256.


556. Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015;61:1579-1590.


558. Ko SE, Lee MW, Rhim H, Kang TW, Song KD, Cha DI, et al. Comparison of procedure-related complications between percutaneous cryoablation and radiofrequency ablation for treating periductal hepatocellular carcinoma. Int J Hyperthermia 2020;37:1354-1361.


560. Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014;270:900-909.


561. Rossi S, Ravetta V, Rosa L, Ghittoni G, Viera FT, Garbagnati F, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology 2011;53:136-147.


562. Imai K, Beppu T, Chikamoto A, Mima K, Okabe H, Hayashi H, et al. Salvage treatment for local recurrence of hepatocellular carcinoma after local ablation therapy. Hepatol Res 2014;44:E335-E345.


565. Sotiropoulos GC, Lang H, Frilling A, Molmenti EP, Paul A, Nadalin S, et al. Resectability of hepatocellular carcinoma: evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepatogastroenterology 2006;53:322-329.

568. Matsui O, Kadoya M, Yoshikawa J, Gabata T, Arai K, Demachi H, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology 1993;188:79-83.


569. Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol 2007;18:365-376.


570. Golfieri R, Cappelli A, Cucchetti A, Piscaglia F, Carpenzano M, Peri E, et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology 2011;53:1580-1589.


572. Golfieri R, Renzulli M, Mosconi C, Forlani L, Giampalma E, Piscaglia F, et al. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Interv Radiol 2013;24:509-517.


573. Iwazawa J, Ohue S, Hashimoto N, Muramoto O, Mitani T. Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. Eur J Radiol 2012;81:3985-3992.


575. Pung L, Ahmad M, Mueller K, Rosenberg J, Stave C, Hwang GL, et al. The role of cone-beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Vasc Interv Radiol 2017;28:334-341.


576. Wattanasatesiri T, Kim HC, Choi JW, Lee JH, Joo I, Hur S, et al. Cone-beam CT-guided chemoembolization in patients with complete response after previous chemoembolization but subsequent elevated ╬▒-fetoprotein without overt hepatocellular carcinoma. J Vasc Interv Radiol 2019;30:1273-1280.


579. Llovet JM, Real MI, Monta├▒a X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-1739.


581. Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461-469.


582. Takayasu K, Arii S, Kudo M, Ichida T, Matsui O, Izumi N, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol 2012;56:886-892.


583. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology 2016;64:106-116.


584. Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai H, Kim JK, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol 2013;24:490-500.


585. Lee HS, Kim KM, Yoon JH, Lee TR, Suh KS, Lee KU, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. J Clin Oncol 2002;20:4459-4465.


586. Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol 2012;81:1173-1178.


590. Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol 1995;165:315-321.


591. Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer 1997;79:2087-2094.


592. Georgiades CS, Hong K, DŌĆÖAngelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2005;16:1653-1659.


593. Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011;258:627-634.


594. Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, Mogal H, et al. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford) 2017;19:659-666.


600. Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol 2015;26:320-329.e6.


602. Yoo DJ, Kim KM, Jin YJ, Shim JH, Ko GY, Yoon HK, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol 2011;26:145-154.


603. Jung SM, Jang JW, You CR, Yoo SH, Kwon JH, Bae SH, et al. Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol 2012;27:684-689.


608. Chung JW, Park JH, Han JK, Choi BI, Han MC, Lee HS, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology 1996;198:33-40.


610. Yang H, Seon J, Sung PS, Oh JS, Lee HL, Jang B, et al. Dexamethasone prophylaxis to alleviate postembolization syndrome after transarterial chemoembolization for hepatocellular carcinoma: a randomized, double-blinded, placebo-controlled study. J Vasc Interv Radiol 2017;28:1503-1511.e2.


612. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol 2007;46:474-481.


614. Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2011;22:1545-1552.


617. Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, et al. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol 2017;28:502-512.


620. Deipolyi AR, Oklu R, Al-Ansari S, Zhu AX, Goyal L, Ganguli S. Safety and efficacy of 70-150 ╬╝m and 100-300 ╬╝m drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2015;26:516-522.


621. Urbano J, Echevarria-Uraga JJ, Ciampi-Dopazo JJ, S├Īnchez-Corral JA, Cobos Alonso J, Anton-Ladislao A, et al. Multicentre prospective study of drug-eluting bead chemoembolisation safety using tightly calibrated small microspheres in non-resectable hepatocellular carcinoma. Eur J Radiol 2020;126:108966.


622. Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol 2011;197:W562-W570.


624. Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol 2006;17:1251-1278.

625. Gabr A, Ranganathan S, Mouli SK, Riaz A, Gates VL, Kulik L, et al. Streamlining radioembolization in UNOS T1/T2 hepatocellular carcinoma by eliminating lung shunt estimation. J Hepatol 2020;72:1151-1158.


626. Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57:1826-1837.


627. Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64.


628. Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-878.


629. Hilgard P, Hamami M, Fouly AE, Scherag A, M├╝ller S, Ertle J, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52:1741-179.


633. Casadei Gardini A, Tamburini E, I├▒arrairaegui M, Frassineti GL, Sangro B. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: a meta-analysis of randomized trials. Onco Targets Ther 2018;11:7315-7321.


634. Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol 2018;36:1913-1921.

635. Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624-1636.

636. Ricke J, Kl├╝mpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019;71:1164-1174.


639. Garin E, Tselikas L, Guiu B, Chalaye J, Edeline J, de Baere T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:17-29.


640. Kim HC, Kim YJ, Lee JH, Suh KS, Chung JW. Feasibility of boosted radioembolization for hepatocellular carcinoma larger than 5 cm. J Vasc Interv Radiol 2019;30:1-8.


641. Kim J, Kim JY, Lee JH, Sinn DH, Hur MH, Hong JH, et al. Long-term outcomes of transarterial radioembolization for large single hepatocellular carcinoma: a comparison to resection. J Nucl Med 2022;63:1215-1222.


642. Benguerfi S, Estrade F, Lescure C, Rolland Y, Palard X, Le Sourd S, et al. Selective internal radiation therapy in older patients with hepatocellular carcinoma: a retrospective analysis. Eur J Gastroenterol Hepatol 2022;34:417-421.


643. Das A, Gabr A, OŌĆÖBrian DP, Riaz A, Desai K, Thornburg B, et al. Contemporary systematic review of health-related quality of life outcomes in locoregional therapies for hepatocellular carcinoma. J Vasc Interv Radiol 2019;30:1924-1933.e2.


644. Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A, et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer 2008;112:1538-1546.


647. Currie BM, Hoteit MA, Ben-Josef E, Nadolski GJ, Soulen MC. Radioembolization-induced chronic hepatotoxicity: a single-center cohort analysis. J Vasc Interv Radiol 2019;30:1915-1923.


649. Vogl TJ, Trapp M, Schroeder H, Mack M, Schuster A, Schmitt J, et al. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology 2000;214:349-357.


650. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010;52:762-773.


653. Kim HY, Park JW, Joo J, Jung SJ, An S, Woo SM, et al. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol 2012;27:1051-1056.


654. Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, M├╝ller C, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology 2013;57:2261-2273.


655. Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, Bayle O, et al. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol 2015;62:855-862.


661. Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-829.


668. Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol 2016;64:1090-1098.


669. Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:565-575.


670. Kudo M, Cheng AL, Park JW, Park JH, Liang PC, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol 2018;3:37-46.


675. Kim TH, Kim DY, Park JW, Kim SH, Choi JI, Kim HB, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2007;67:225-231.


676. Schefter TE, Kavanagh BD, Timmerman RD, Cardenes HR, Baron A, Gaspar LE. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005;62:1371-1378.


678. Kimura T, Takeda A, Tsurugai Y, Kawano R, Doi Y, Oku Y, et al. A multi-institutional retrospective study of repeated stereotactic body radiation therapy for intrahepatic recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2020;108:1265-1275.


679. McDuff SGR, Remillard KA, Zheng H, Raldow AC, Wo JY, Eyler CE, et al. Liver reirradiation for patients with hepatocellular carcinoma and liver metastasis. Pract Radiat Oncol 2018;8:414-421.


680. Oshiro Y, Mizumoto M, Okumura T, Fukuda K, Fukumitsu N, Abei M, et al. Analysis of repeated proton beam therapy for patients with hepatocellular carcinoma. Radiother Oncol 2017;123:240-245.


682. Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447-e453.


684. Sanuki N, Takeda A, Oku Y, Mizuno T, Aoki Y, Eriguchi T, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol 2014;53:399-404.


688. Takeda A, Sanuki N, Tsurugai Y, Iwabuchi S, Matsunaga K, Ebinuma H, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 2016;122:2041-2049.


690. Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol 2005;23:1839-1846.


691. Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2009;74:831-836.


693. Nakayama H, Sugahara S, Tokita M, Fukuda K, Mizumoto M, Abei M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer 2009;115:5499-5506.


694. Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer 2011;117:4890-4904.


695. Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer 2011;117:3053-3059.


698. Bush DA, Smith JC, Slater JD, Volk ML, Reeves ME, Cheng J, et al. Randomized clinical trial comparing proton beam radiation therapy with transarterial chemoembolization for hepatocellular carcinoma: results of an interim analysis. Int J Radiat Oncol Biol Phys 2016;95:477-482.


702. Kim JW, Kim DY, Han KH, Seong J. Phase I/II trial of helical IMRT-based stereotactic body radiotherapy for hepatocellular carcinoma. Dig Liver Dis 2019;51:445-451.


704. Durand-Labrunie J, Baumann AS, Ayav A, Laurent V, Boleslawski E, Cattan S, et al. Curative irradiation treatment of hepatocellular carcinoma: a multicenter phase 2 trial. Int J Radiat Oncol Biol Phys 2020;107:116-125.


705. Beaton L, Dunne EM, Yeung R, Rackley T, Weber B, Mar C, et al. Stereotactic body radiotherapy for large unresectable hepatocellular carcinomas - a single institution phase II study. Clin Oncol (R Coll Radiol) 2020;32:423-432.


711. Iwata H, Ogino H, Hattori Y, Nakajima K, Nomura K, Hashimoto S, et al. A phase 2 study of image-guided proton therapy for operable or ablation-treatable primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2021;111:117-126.


712. Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol 2015;1:756-765.


713. Hsu HC, Chen TY, Chiu KW, Huang EY, Leung SW, Huang YJ, et al. Three-dimensional conformal radiotherapy for the treatment of arteriovenous shunting in patients with hepatocellular carcinoma. Br J Radiol 2007;80:38-42.


714. Oh D, Lim DH, Park HC, Paik SW, Koh KC, Lee JH, et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol 2010;33:370-375.

715. Choi C, Koom WS, Kim TH, Yoon SM, Kim JH, Lee HS, et al. A prospective phase 2 multicenter study for the efficacy of radiation therapy following incomplete transarterial chemoembolization in unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2014;90:1051-1060.


718. Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2003;57:113-119.


719. Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer 2005;103:2419-2246.


720. Han KH, Seong J, Kim JK, Ahn SH, Lee DY, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer 2008;113:995-1003.


721. Shirai S, Sato M, Suwa K, Kishi K, Shimono C, Sonomura T, et al. Feasibility and efficacy of single photon emission computed tomography-based three-dimensional conformal radiotherapy for hepatocellular carcinoma 8 cm or more with portal vein tumor thrombus in combination with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys 2010;76:1037-1044.


722. Koo JE, Kim JH, Lim YS, Park SJ, Won HJ, Sung KB, et al. Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys 2010;78:180-187.


724. Chuma M, Taguchi H, Yamamoto Y, Shimizu S, Nakanishi M, Ogawa K, et al. Efficacy of therapy for advanced hepatocellular carcinoma: intra-arterial 5-fluorouracil and subcutaneous interferon with image-guided radiation. J Gastroenterol Hepatol 2011;26:1123-1132.


725. Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys 2012;82:2004-2011.


726. Hou JZ, Zeng ZC, Zhang JY, Fan J, Zhou J, Zeng MS. Influence of tumor thrombus location on the outcome of external-beam radiation therapy in advanced hepatocellular carcinoma with macrovascular invasion. Int J Radiat Oncol Biol Phys 2012;84:362-368.


730. Kim DY, Park JW, Kim TH, Kim BH, Moon SH, Kim SS, et al. Risk-adapted simultaneous integrated boost-proton beam therapy (SIB-PBT) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother Oncol 2017;122:122-129.


735. Rim CH, Jeong BK, Kim TH, Kim JH, Kang HC, Seong J. Effectiveness and feasibility of external beam radiotherapy for hepatocellular carcinoma with inferior vena cava and/or right atrium involvement: a multicenter trial in Korea (KROG 17-10). Int J Radiat Biol 2020;96:759-766.


736. Rim CH, Kim CY, Yang DS, Yoon WS. External beam radiation therapy to hepatocellular carcinoma involving inferior vena cava and/or right atrium: a meta-analysis and systemic review. Radiother Oncol 2018;129:123-129.


737. Kim BK, Kim DY, Byun HK, Choi HJ, Beom SH, Lee HW, et al. Efficacy and safety of liver-directed concurrent chemoradiotherapy and sequential sorafenib for advanced hepatocellular carcinoma: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys 2020;107:106-115.


738. Im JH, Yoon SM, Park HC, Kim JH, Yu JI, Kim TH, et al. Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumour thrombosis in a hepatitis B endemic area. Liver Int 2017;37:90-100.


749. Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: a retrospective cohort study. Int J Surg 2017;44:223-228.


754. Katz AW, Chawla S, Qu Z, Kashyap R, Milano MT, Hezel AF. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys 2012;83:895-900.


755. Mannina EM, Cardenes HR, Lasley FD, Goodman B, Zook J, Althouse S, et al. Role of stereotactic body radiation therapy before orthotopic liver transplantation: retrospective evaluation of pathologic response and outcomes. Int J Radiat Oncol Biol Phys 2017;97:931-938.


757. Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2005;61:1143-1150.


758. Kim TH, Kim DY, Park JW, Kim YI, Kim SH, Park HS, et al. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol 2006;29:568-575.


759. Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;84:355-361.


760. Seong J, Park HC, Han KH, Lee DY, Lee JT, Chon CY, et al. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys 2000;47:1331-1335.


761. Bae SH, Park HC, Lim DH, Lee JA, Gwak GY, Choi MS, et al. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;82:e603-e607.


763. Soliman H, Ringash J, Jiang H, Singh K, Kim J, Dinniwell R, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013;31:3980-3986.


765. Cheng SH, Lin YM, Chuang VP, Yang PS, Cheng JC, Huang AT, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol 1999;14:1025-1033.


766. Huang JF, Wang LY, Lin ZY, Chen SC, Hsieh MY, Chuang WL, et al. Incidence and clinical outcome of icteric type hepatocellular carcinoma. J Gastroenterol Hepatol 2002;17:190-195.


768. Zeng ZC, Tang ZY, Fan J, Qin LX, Ye SL, Zhou J, et al. Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys 2005;63:1067-1076.


770. Yamashita H, Nakagawa K, Shiraishi K, Tago M, Igaki H, Nakamura N, et al. Radiotherapy for lymph node metastases in patients with hepatocellular carcinoma: retrospective study. J Gastroenterol Hepatol 2007;22:523-527.


771. Jang JW, Kay CS, You CR, Kim CW, Bae SH, Choi JY, et al. Simultaneous multitarget irradiation using helical tomotherapy for advanced hepatocellular carcinoma with multiple extrahepatic metastases. Int J Radiat Oncol Biol Phys 2009;74:412-418.


776. Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int 2005;25:261-265.


777. He J, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer 2009;115:2710-2720.


778. Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N, Fukushima S, Saito T. Radiation therapy and palliative care prolongs the survival of hepatocellular carcinoma patients with bone metastases. Intern Med 2016;55:1077-1083.


781. Rades D, Dahlke M, Janssen S, Gebauer N, Bartscht T. Radiation therapy for metastatic spinal cord compression in patients with hepatocellular carcinoma. In Vivo 2015;29:749-752.

785. Kim N, Kim HJ, Won JY, Kim DY, Han KH, Jung I, et al. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol 2019;131:81-87.


787. Kim N, Cheng J, Jung I, Liang J, Shih YL, Huang WY, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol 2020;73:121-129.


790. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390.


791. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34.


792. Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 2013;31:4067-4075.


793. Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31:3517-3524.


795. Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015;33:559-566.


796. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-1173.


797. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894-1905.


798. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2022;23:77-90.


799. Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci 2008;99:159-165.


802. Hollebecque A, Cattan S, Romano O, Sergent G, Mourad A, Louvet A, et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Aliment Pharmacol Ther 2011;34:1193-1201.


803. Chiu J, Tang YF, Yao TJ, Wong A, Wong H, Leung R, et al. The use of single-agent sorafenib in the treatment of advanced hepatocellular carcinoma patients with underlying Child-Pugh B liver cirrhosis: a retrospective analysis of efficacy, safety, and survival benefits. Cancer 2012;118:5293-5301.


804. Pressiani T, Boni C, Rimassa L, Labianca R, Fagiuoli S, Salvagni S, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol 2013;24:406-411.


806. Suzuki E, Kaneko S, Okusaka T, Ikeda M, Yamaguchi K, Sugimoto R, et al. A multicenter phase II study of sorafenib in Japanese patients with advanced hepatocellular carcinoma and Child Pugh A and B class. Jpn J Clin Oncol 2018;48:317-321.


808. Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol 2016;65:1140-1147.


809. Brose MS, Frenette CT, Keefe SM, Stein SM. Management of sorafenib-related adverse events: a clinicianŌĆÖs perspective. Semin Oncol 2014;41 Suppl 2:S1-S16.


811. Ren Z, Zhu K, Kang H, Lu M, Qu Z, Lu L, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015;33:894-900.


812. Lee YS, Jung YK, Kim JH, Cho SB, Kim DY, Kim MY, et al. Effect of urea cream on sorafenib-associated hand-foot skin reaction in patients with hepatocellular carcinoma: a multicenter, randomised, double-blind controlled study. Eur J Cancer 2020;140:19-27.


815. Jannin A, Penel N, Ladsous M, Vantyghem MC, Do Cao C. Tyrosine kinase inhibitors and immune checkpoint inhibitors-induced thyroid disorders. Crit Rev Oncol Hematol 2019;141:23-35.


824. Ogushi K, Chuma M, Uojima H, Hidaka H, Numata K, Kobayashi S, et al. Safety and ef’¼ücacy of lenvatinib treatment in Child-Pugh A and B patients with unresectable hepatocellular carcinoma in clinical practice: a multicenter analysis. Clin Exp Gastroenterol 2020;13:385-396.


828. Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol 2022;4_suppl:379.

830. Kelley RK, Yau T, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. VP10-2021: cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): results from the randomized phase III COSMIC-312 trial. Ann Oncol 2022;33:114-116.

831. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-873.


834. Rimassa L, Personeni N, Czauderna C, Foerster F, Galle P. Systemic treatment of HCC in special populations. J Hepatol 2021;74:931-943.


835. Pfister D, N├║├▒ez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021;592:450-456.


836. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol 2020;38:193-202.


837. Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol 2017;35:622-628.


838. Reig M, Rimola J, Torres F, Darnell A, Rodriguez-Lope C, Forner A, et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology 2013;58:2023-2031.


840. Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509-3516.


841. Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67.


842. Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-870.


843. Rimassa L, Assenat E, Peck-Radosavljevic M, Pracht M, Zagonel V, Mathurin P, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol 2018;19:682-693.


844. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66.


846. Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE-394 study. J Clin Oncol 2022;4_suppl:383.

847. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased ╬▒-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282-296.


850. Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Sch├╝tz G, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-255.


854. Kudo M, Matilla A, Santoro A, Melero I, Graci├Īn AC, Acosta-Rivera M, et al. CheckMate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol 2021;75:600-609.


856. Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol 2021;6:559-568.


857. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21:571-580.


867. Thomas MB. Systemic therapy for hepatocellular carcinoma. Cancer J 2008;14:123-127.


868. Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, et al. Oxaliplatin-based chemotherapy: a new option in advanced hepatocellular carcinoma. a systematic review and pooled analysis. Clin Oncol (R Coll Radiol) 2014;26:488-496.


869. Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013;31:3501-3508.


871. Lim TY, Cheong JY, Cho SW, Sim SJ, Kim JS, Choi SJ, et al. Effect of low dose 5-fluorouracil and cisplatin intra-arterial infusion chemotherapy in advanced hepatocellular carcinoma with decompensated cirrhosis. Korean J Hepatol 2006;12:65-73.

873. Hamada A, Yamakado K, Nakatsuka A, Takaki H, Akeboshi M, Takeda K. Hepatic arterial infusion chemotherapy with use of an implanted port system in patients with advanced hepatocellular carcinoma: prognostic factors. J Vasc Interv Radiol 2004;15:835-841.


876. Kawaoka T, Aikata H, Hyogo H, Morio R, Morio K, Hatooka M, et al. Comparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinoma. J Dig Dis 2015;16:505-512.


878. Terashima T, Yamashita T, Arai K, Sunagozaka H, Kitahara M, Nakagawa H, et al. Feasibility and efficacy of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma after sorafenib. Hepatol Res 2014;44:1179-1185.


879. Jeong SW, Jang JY, Lee JE, Lee SH, Kim SG, Cha SW, et al. The efficacy of hepatic arterial infusion chemotherapy as an alternative to sorafenib in advanced hepatocellular carcinoma. Asia Pac J Clin Oncol 2012;8:164-171.


883. Lyu N, Wang X, Li JB, Lai JF, Chen QF, Li SL, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol 2022;40:468-480.


886. Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol 2018;3:424-432.


889. Zheng K, Zhu X, Fu S, Cao G, Li WQ, Xu L, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology 2022;303:455-464.


892. Li QJ, He MK, Chen HW, Fang WQ, Zhou YM, Xu L, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol 2022;40:150-160.


893. Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol 2019;70:684-691.


894. Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol 2022 Aug 3;doi:
10.1200/JCO.22.00392.

895. Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol 2018;36:1668-1674.


899. Liao M, Zhu Z, Wang H, Huang J. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: a meta-analysis. Scand J Gastroenterol 2017;52:624-634.


902. Chu KJ, Lai EC, Yao XP, Zhang HW, Lau WY, Fu XH, et al. Vitamin analogues in chemoprevention of hepatocellular carcinoma after resection or ablation--a systematic review and meta-analysis. Asian J Surg 2010;33:120-126.


904. Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-1354.


906. Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000;356:802-807.


908. Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015;148:1383-1391.e6.


910. Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis 2009;41:36-41.


911. Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother 2008;31:63-71.


914. Wang H, Liu A, Bo W, Feng X, Hu Y, Tian L, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma patients after curative resection, a systematic review and meta-analysis. Dig Liver Dis 2016;48:1275-1282.


915. Yeo W, Lam KC, Zee B, Chan PS, Mo FK, Ho WM, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol 2004;15:1661-1666.


916. Nagamatsu H, Itano S, Nagaoka S, Akiyoshi J, Matsugaki S, Kurogi J, et al. Prophylactic lamivudine administration prevents exacerbation of liver damage in HBe antigen positive patients with hepatocellular carcinoma undergoing transhepatic arterial infusion chemotherapy. Am J Gastroenterol 2004;99:2369-2375.


920. Park JW, Park KW, Cho SH, Park HS, Lee WJ, Lee DH, et al. Risk of hepatitis B exacerbation is low after transcatheter arterial chemoembolization therapy for patients with HBV-related hepatocellular carcinoma: report of a prospective study. Am J Gastroenterol 2005;100:2194-2200.


922. Lao XM, Wang D, Shi M, Liu G, Li S, Guo R, et al. Changes in hepatitis B virus DNA levels and liver function after transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatol Res 2011;41:553-563.


923. Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology 2006;43:233-240.


925. Li X, Zhong X, Chen ZH, Wang TT, Ma XK, Xing YF, et al. Efficacy of prophylactic entecavir for hepatitis B virus-related hepatocellular carcinoma receiving transcatheter arterial chemoembolization. Asian Pac J Cancer Prev 2015;16:8665-8670.


927. Tamori A, Nishiguchi S, Tanaka M, Kurooka H, Fujimoto S, Nakamura K, et al. Lamivudine therapy for hepatitis B virus reactivation in a patient receiving intra-arterial chemotherapy for advanced hepatocellular carcinoma. Hepatol Res 2003;26:77-80.


931. Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Ann Surg 2015;261:56-66.

932. Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2007;69:813-819.


935. Dan JQ, Zhang YJ, Huang JT, Chen MS, Gao HJ, Peng ZW, et al. Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol 2013;39:865-872.


941. Carr BI, Pujol L. Pain at presentation and survival in hepatocellular carcinoma. J Pain 2010;11:988-993.


942. Ibrahim NM, Abdelhameed KM, Kamal SMM, Khedr EMH, Kotb HIM. Effect of transcranial direct current stimulation of the motor cortex on visceral pain in patients with hepatocellular carcinoma. Pain Med 2018;19:550-560.


944. Hong SH, Roh SY, Kim SY, Shin SW, Kim CS, Choi JH, et al. Change in cancer pain management in Korea between 2001 and 2006: results of two nationwide surveys. J Pain Symptom Manage 2011;41:93-103.


946. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437-1449.


949. Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721-1730.


950. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-742.


951. Ryu E, Kim K, Cho MS, Kwon IG, Kim HS, Fu MR. Symptom clusters and quality of life in Korean patients with hepatocellular carcinoma. Cancer Nurs 2010;33:3-10.


953. Radner H, Ramiro S, Buchbinder R, Landew├® RB, van der Heijde D, Aletaha D. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev 2012;1:CD008951.


954. World Health Organization (WHO). Cancer pain relief: with a guide to opioid availability. Geneva: WHO; 1996.
955. Ministry of Health & Welfare. Cancer pain management guideline. Seoul: Ministry of Health & Welfare; 2012.
956. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guideline in oncology: adult cancer pain. Vol. 1. Fort Washington: NCCN; 2013.
957. Rossi S, Assis DN, Awsare M, Brunner M, Skole K, Rai J, et al. Use of over-the-counter analgesics in patients with chronic liver disease: physiciansŌĆÖ recommendations. Drug Saf 2008;31:261-270.

958. Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005;42:1364-1372.


959. UFaDAF UDoHaHS. Drugs: acetaminophen information. Washington, D.C.: US Department of Health and Human Services; 2017.
960. Mofredj A, Cadranel JF, Darchy B, Barbare JC, Cazier A, Pras V, et al. Hepatotoxicity caused by therapeutic doses of paracetamol in alcoholics. Report of 2 cases of fatal hepatitis in cirrhosis. Ann Med Interne (Paris) 1999;150:507-511.

961. Dart RC, Bailey E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy 2007;27:1219-1230.


963. Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther 2007;26:283-290.


965. Villeneuve JP, Raymond G, Bruneau J, Colpron L, Pomier-Layrargues G. Pharmacokinetics and metabolism of acetaminophen in normal, alcoholic and cirrhotic subjects. Gastroenterol Clin Biol 1983;7:898-902.

966. Hirschfield GM, Kumagi T, Heathcote EJ. Preventative hepatology: minimising symptoms and optimising care. Liver Int 2008;28:922-934.


967. Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther 2005;12:133-141.


971. Riley TR 3rd, Smith JP. Ibuprofen-induced hepatotoxicity in patients with chronic hepatitis C: a case series. Am J Gastroenterol 1998;93:1563-1565.


972. Ackerman Z, Cominelli F, Reynolds TB. Effect of misoprostol on ibuprofen-induced renal dysfunction in patients with decompensated cirrhosis: results of a double-blind placebo-controlled parallel group study. Am J Gastroenterol 2002;97:2033-2039.


973. Castro-Fern├Īndez M, S├Īnchez-Mu├▒oz D, Gal├Īn-Jurado MV, Larraona JL, Su├Īrez E, Lamas E, et al. Influence of nonsteroidal antiinflammatory drugs in gastrointestinal bleeding due to gastroduodenal ulcers or erosions in patients with liver cirrhosis. Gastroenterol Hepatol 2006;29:11-14.

974. Lee YC, Chang CH, Lin JW, Chen HC, Lin MS, Lai MS. Non-steroidal anti-inflammatory drugs use and risk of upper gastrointestinal adverse events in cirrhotic patients. Liver Int 2012;32:859-866.


979. Tegeder I, L├Čtsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet 1999;37:17-40.


980. Kotb HI, El-Kady SA, Emara SE, Fouad EA, El-Kabsh MY. Pharmacokinetics of controlled release morphine (MST) in patients with liver carcinoma. Br J Anaesth 2005;94:95-99.


981. Tallgren M, Olkkola KT, Sepp├żl├ż T, H├Čckerstedt K, Lindgren L. Pharmacokinetics and ventilatory effects of oxycodone before and after liver transplantation. Clin Pharmacol Ther 1997;61:655-661.


982. Durnin C, Hind ID, Ghani SP, Yates DB, Molz KH. Pharmacokinetics of oral immediate-release hydromorphone (Dilaudid IR) in subjects with moderate hepatic impairment. Proc West Pharmacol Soc 2001;44:83-84.

983. Haberer JP, Schoeffler P, Couderc E, Duvaldestin P. Fentanyl pharmacokinetics in anaesthetized patients with cirrhosis. Br J Anaesth 1982;54:1267-1270.


984. Berry P, Kotha S. Improving end of life care in liver disease. J Hepatol 2022;76:1225-1226.


985. Kashima M, Yamakado K, Takaki H, Kaminou T, Tanigawa N, Nakatsuka A, et al. Radiofrequency ablation for the treatment of bone metastases from hepatocellular carcinoma. AJR Am J Roentgenol 2010;194:536-541.


987. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207-214.


988. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-216.

989. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-247.


990. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60.


991. Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 2009;115:616-623.


992. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-430.

993. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698-711.


999. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol 2020;72:288-306.


1001. Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol 2017;66:1166-1172.


1002. Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol 2019;70:1262-1277.


1004. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-952.

1006. Hodi FS, Ballinger M, Lyons B, Soria JC, Nishino M, Tabernero J, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 2018;36:850-858.


1007. Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 2018;88:38-47.


1008. Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol 2009;16:792-794.


1009. Liu D, Chan AC, Fong DY, Lo CM, Khong PL. Evidence-based surveillance imaging schedule after liver transplantation for hepatocellular carcinoma recurrence. Transplantation 2017;101:107-111.


1012. Zheng J, Chou JF, G├Čnen M, Vachharajani N, Chapman WC, Majella Doyle MB, et al. Prediction of hepatocellular carcinoma recurrence beyond Milan criteria after resection: validation of a clinical risk score in an international cohort. Ann Surg 2017;266:693-701.

1013. Dioguardi Burgio M, Ronot M, Fuks D, Dondero F, Cauchy F, Gaujoux S, et al. Follow-up imaging after liver transplantation should take into consideration primary hepatocellular carcinoma characteristics. Transplantation 2015;99:1613-1618.


1022. National Comprehensive Cancer Network (NCCN). NCCN: cancer and COVID-19 vaccination version 7.0. Plymouth Meeting: NCCN; 2022.
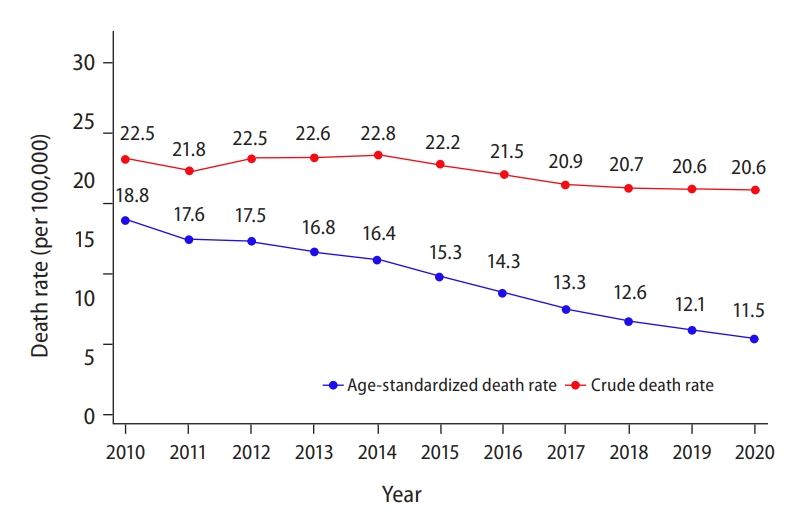


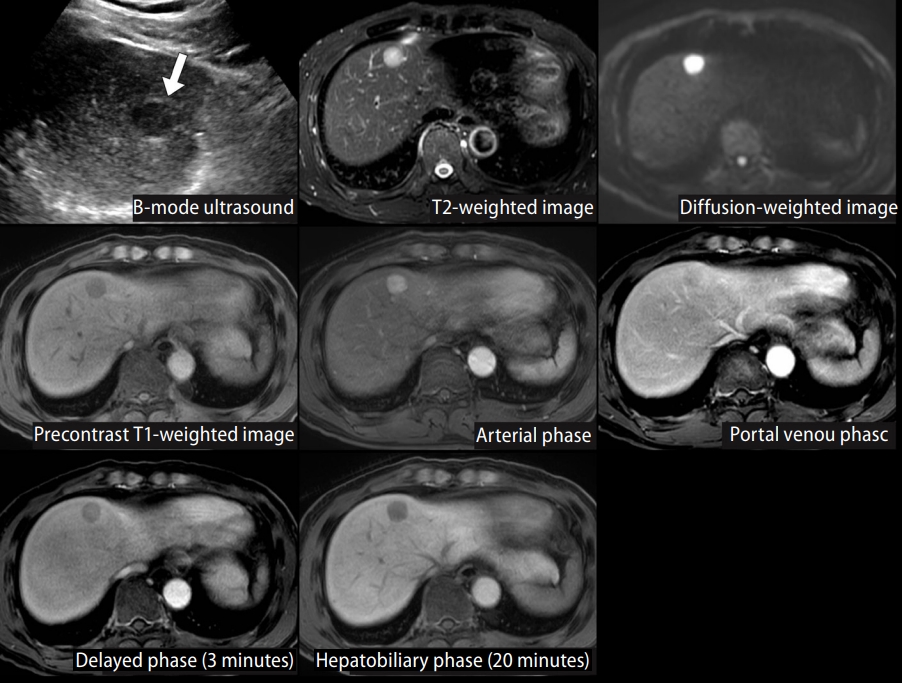



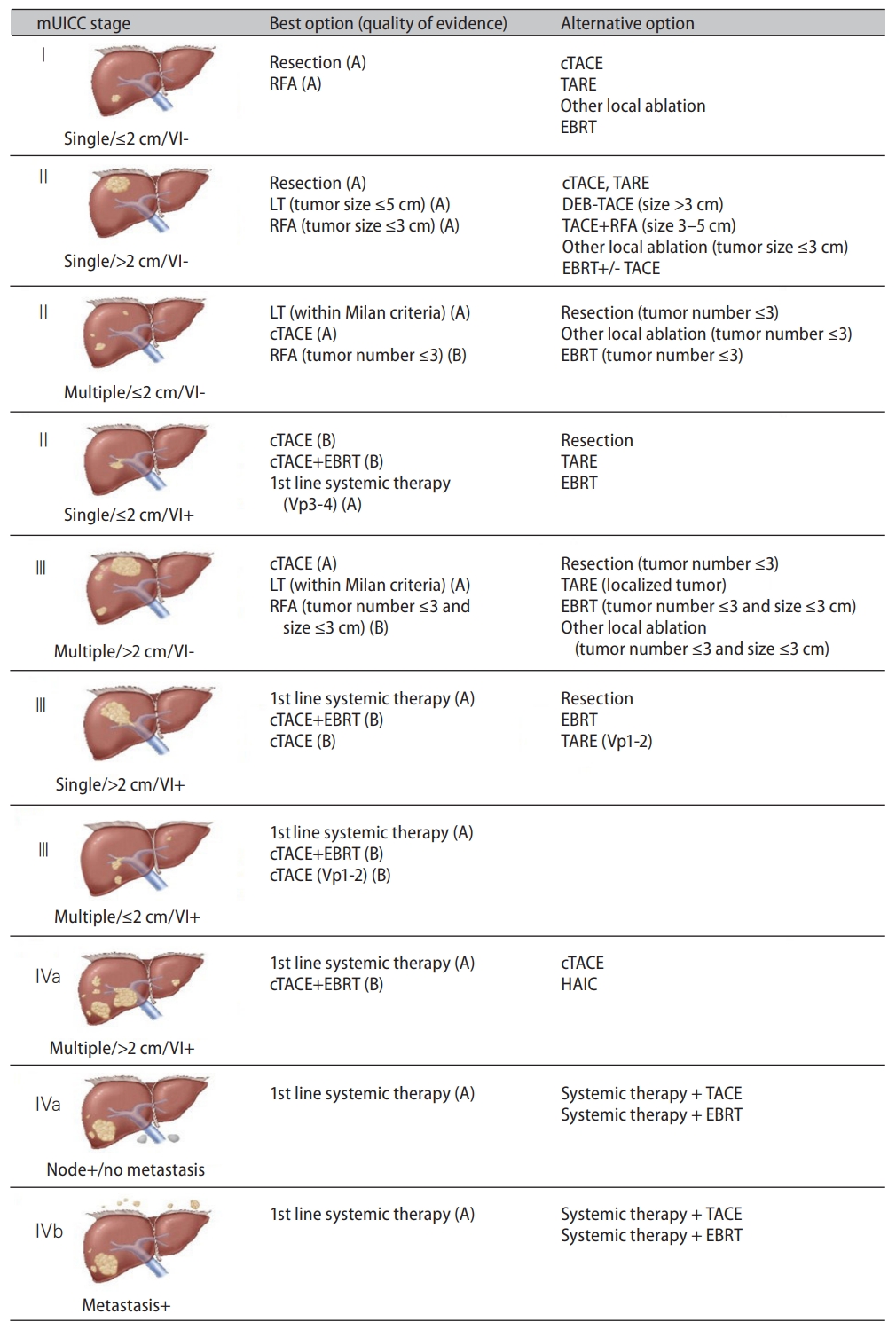
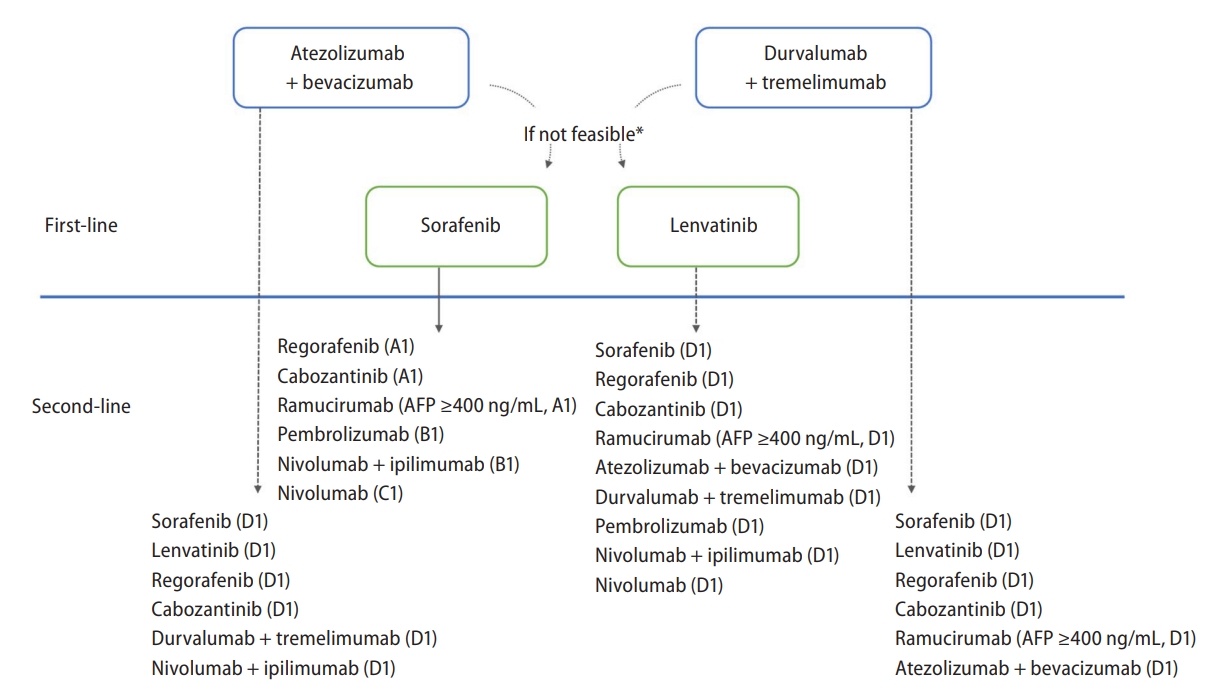
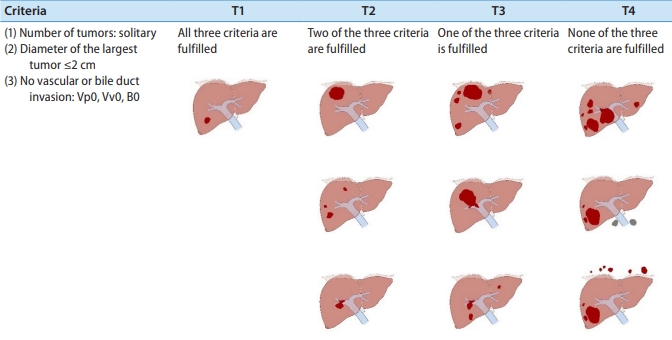



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




