| Clin Mol Hepatol > Volume 23(4); 2017 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
- Study design: Asma Siddique, and Hyun Phil Shin
- Acquisition and interpretation of data: Asma Siddique, Blaire Burman, Hyun Phil Shin
- Drafting of the manuscript: Asma Siddique, Richard A. Kozarek
- Critical revision of the manuscript for important intellectual content: Asma Siddique, and Hyun Phil Shin
- Statistical analysis: Ji-Ae Park
Figure┬Ā1.
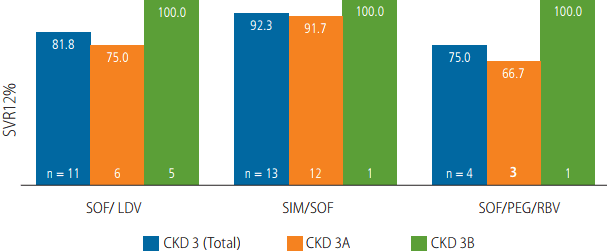
Figure┬Ā2.

Table┬Ā1.
| Baseline characteristic | CKD 3A (n=21) | CKD 3B (n=7) | All patients (n=28) | P-value |
|---|---|---|---|---|
| Treatment regimen (n, %) | 0.097* | |||
| ŌĆāSOF/LDV | 6 (28.6) | 5 (71.4) | 11 (39.3) | |
| ŌĆāSOF/SIM | 12 (57.1) | 1 (14.3) | 13 (46.4) | |
| ŌĆāSOF/PEG/RBV | 3 (14.3) | 1 (14.3) | 4 (14.3) | |
| Age (mean┬▒SD, range), years | 61.0 ┬▒ 11.2 (27-78) | 62.9 ┬▒ 5.4 (56-72) | 61.4 ┬▒ 10.0 (27-78) | 0.67 |
| Male Sex (n, %) | 19 (47.6) | 5 (71.4) | 15 (53.6) | 0.253* |
| Body-mass index (mean┬▒SD, range) | 28.3 ┬▒ 4.2 (21-36.7) | 25.5 ┬▒ 2.6 (23.2-30.9) | 27.6 ┬▒ 4.0 (21-36.7) | 0.113 |
| Race (n, %) | 0.253* | |||
| ŌĆāWhite | 19 (90.5) | 5 (71.4) | 24 (85.7) | |
| ŌĆāAfrican American | 1 (4.8) | 1 (14.3) | 2 (7.1) | |
| ŌĆāOther | 1 (4.8) | 1 (14.3) | 2 (7.1) | |
| HCV genotype (n, %) | 0.674* | |||
| ŌĆā1a | 14 (66.7) | 4 (57.1) | 18 (64.3) | |
| ŌĆā1b | 7 (33.3) | 3 (42.9) | 5 (31) | |
| Previous HCV treatment (n, %) | 0.212* | |||
| ŌĆāNa├»ve | 12 (57.1) | 5 (72.4) | 17 (60.7) | |
| ŌĆāTreatment experienced (IFN based) (n, %) | 9 (42.9) | 2 (28.6) | 11 (39.3) | |
| Cirrhosis (n, %) | 11 (52.4) | 3 (42.9) | 14(50.0) | 1* |
| Diabetes (n, %) | 4 (19.0) | 4 (57.1) | 8 (28.6) | 0.142* |
| Pre-laboratory test (mean┬▒SD, range) | ||||
| ŌĆāHCV RNA (log10 U/L) | 6.1 ┬▒ 0.9 (2.8-7.0) | 6.1 ┬▒ 0.6 (5.2-6.8) | 6.1 ┬▒ 0.0 (2.8-7.0) | 0.841 |
| ŌĆāHb (g/dL) | 13.5 ┬▒ 2.0 (8.6-16.1) | 13.3 ┬▒ 2.3 (11.0-16.5) | 13.4 ┬▒ 2.0 (8.6-16.5) | 0.853 |
| ŌĆāPlatelet (109/L) | 144.1 ┬▒ 72.5 (29-284) | 153.4 ┬▒ 44.0 (85-214) | 146.5 ┬▒ 65.9 (29-284) | 0.754 |
| ŌĆāPT (INR) | 1.14 ┬▒ 0.16 (0.9-1.4) | 1.04 ┬▒ 0.09 (1.0-1.2) | 1.12 ┬▒ 0.03 (0.9-1.4) | 0.195 |
| ŌĆāAlbumin (g/dL) | 3.7 ┬▒ 0.6 (2.0-4.6) | 3.7 ┬▒ 0.4 (3.0-4.2) | 3.7 ┬▒ 0.6 (2.0-4.6) | 0.91 |
| ŌĆāALT (U/L) | 69.2 ┬▒ 53.2 (21-250) | 56.4 ┬▒ 42.2 (6-134) | 66 ┬▒ 50.2 (6-250) | 0.57 |
| ŌĆāAST (U/L) | 62.5 ┬▒ 32.8 (25-160) | 47.3 ┬▒ 27.2 (13-87) | 58.7 ┬▒ 31.7 (13-160) | 0.279 |
| ŌĆāBilirubin (mg/dL) | 0.8 ┬▒ 0.4 (0.3-2.0) | 0.5 ┬▒ 0.2 (0.4-0.8) | 0.7 ┬▒ 0.4 (0.3-2.0) | 0.029 |
| ŌĆāCreatinine (mg/dL) | 1.2 ┬▒ 0.2 (0.9-1.7) | 1.8 ┬▒ 0.3 (1.3-2.2) | 1.3 ┬▒ 0.4 (0.9-2.2) | <0.001 |
| ŌĆāCrCl (mL/min/1.73m2) | 55.2 ┬▒ 3.7 (47.4-59.9) | 36.6 ┬▒ 6.4 (30.0-44.2) | 50.6 ┬▒ 9.3 (30.0-59.9) | <0.001 |
Values are presented as mean ┬▒ SD or n (%) unless otherwise indicated. The body-mass index is the weight in kilograms divided by the square of height in meters.
CKD, chronic kidney disease; CKD 3A, estimated glomerular filtration rate (eGFR) 45ŌĆō60 mL/min/1.73m2; CKD 3B, eGFR 30-45 mL/min/1.73m2; SOF, sofosbuvir; LDV, ledipasvir; SIM, simeprevir; PEG, pegylated interferon; RBV, ribavirin; SD, standard deviation; HCV, hepatitis C virus; IFN, interferon; Hb, hemoglobin; PT, prothrombin time; INR, international normalized ratio; ALT, alanine transaminase; AST, aspartate transaminase; CrCl, creatinine clearance.
Table┬Ā2.
| Response | SVR12 rates-no./ total no. (%) | P-value |
|---|---|---|
| Chronic kidney disease stage | 0.545* | |
| ŌĆā3A | 17/21 (81.0) | |
| ŌĆā3B | 7/7 (100) | |
| Treatment regimen | 0.469* | |
| ŌĆāSOF/LDV | 9/11 (81.8) | |
| ŌĆāSOF/SIM | 12/13 (92.3) | |
| ŌĆāSOF/PEG/RBV | 3/4 (75.0) | |
| Rapid virological responseŌĆĀ | 0.166* | |
| ŌĆāRVR (-) | 3/5 (60.0) | |
| ŌĆāRVR (+) | 18/20 (90.0) | |
| Cirrhosis | 0.596* | |
| ŌĆāCirrhosis (-) | 13/14 (92.9) | |
| ŌĆāCirrhosis (+) | 11/14 (78.6) | |
| DM | 0.555 | |
| ŌĆāDM (-) | 18/20 (90.0) | |
| ŌĆāDM (+) | 6/8 (75.0) | |
| Gender | 1* | |
| ŌĆāMale | 13/15 (86.7) | |
| ŌĆāFemele | 11/13 (84.6) | |
| Age | 0.116* | |
| ŌĆā<65 | 17/18 (94.4) | |
| ŌĆā65 | 7/10 (70.0) | |
| BMI | 1* | |
| ŌĆā<30 | 17/20 (85.0) | |
| ŌĆā30 | 7/8 (87.5) | |
| Pretreatment HCV RNA (IU/mL) | 1* | |
| ŌĆā<800,000 | 6/7 (85.7) | |
| ŌĆāŌēź800,000 | 18/21 (85.7) | |
| Previous treatment | 1* | |
| ŌĆāNa├»ve | 15/17 (88.2) | |
| ŌĆāTreatment experienced | 9/11 (81.8) | |
| HCV genotype (n, %) | 0.116* | |
| ŌĆā1a | 17/18 (94.4) | |
| ŌĆā1b | 7/10 (70.0) | |
| Race (n, %) | 1* | |
| ŌĆāCaucasian | 20/24 (83.3) | |
| ŌĆāAfrican American | 2/2 (100) | |
| ŌĆāOther | 2/2 (100) |
The body-mass index is the weight in kilograms divided by the square of height in meters.
SVR12, sustained virological response 12 weeks post treatment; SOF, sofosbuvir; LDV, ledipasvir; SIM, simeprevir; PEG, pegylated interferon; RBV, ribavirin; RVR, rapid virological response; DM, diabetes mellitus; BMI, body mass index; HCV, hepatitis C virus.
Table┬Ā3.
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Asma Siddique

https://orcid.org/0000-0002-1284-6906 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



