| Clin Mol Hepatol > Volume 30(2); 2024 > Article |
|
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary┬ĀTable┬Ā1.
Supplementary┬ĀTable┬Ā2.
Supplementary┬ĀFig.┬Ā1.
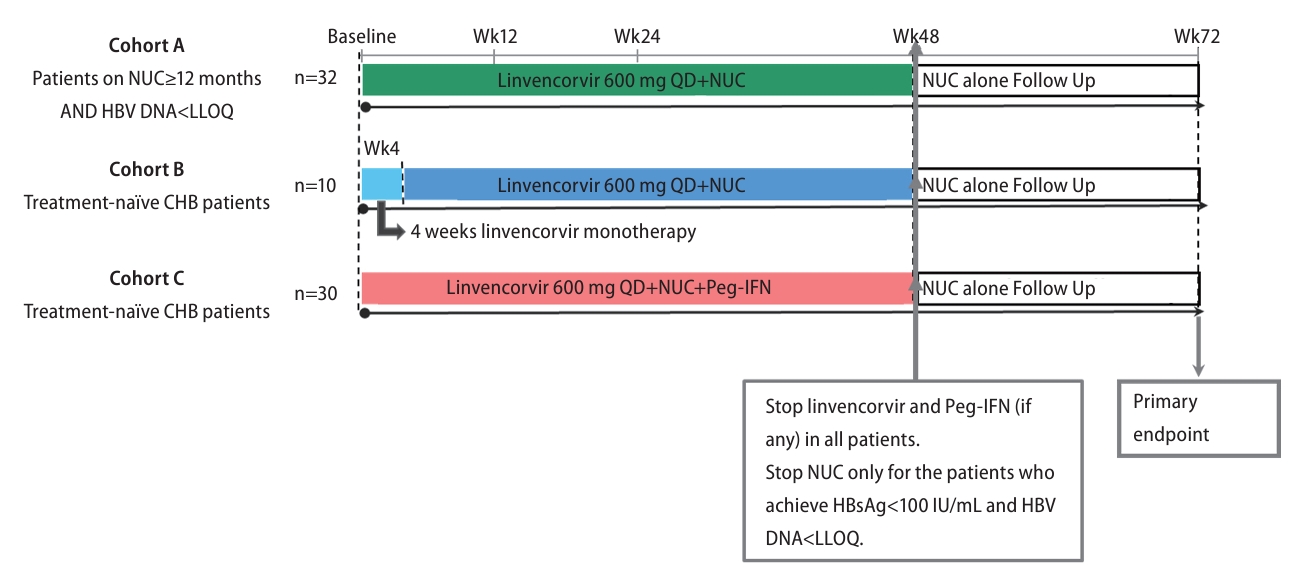
Figure┬Ā1.
Figure┬Ā3.
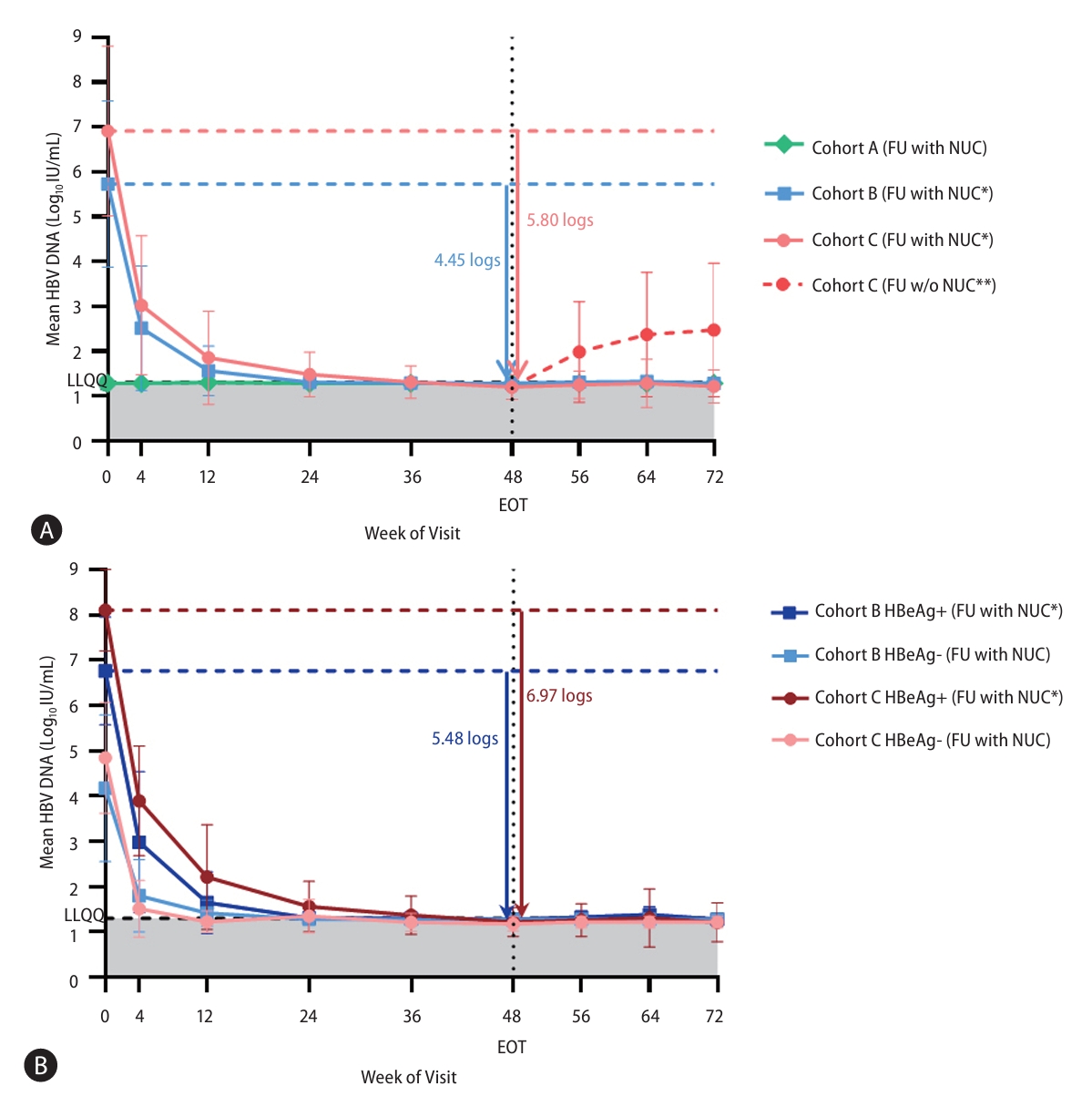
Figure┬Ā4.

Figure┬Ā5.

Table┬Ā1.
| Characteristics | NUC-suppressed Cohort A (n=32) |
Treatment-naïve |
|
|---|---|---|---|
| Cohort B (n=10) | Cohort C (n=30) | ||
| Age, years | 47.2 (8.3) | 43.8 (9.8) | 32.8 (7.7) |
| Sex | |||
| ŌĆāFemale | 13 (41%) | 5 (50%) | 7 (23%) |
| ŌĆāMale | 19 (59%) | 5 (50%) | 23 (77%) |
| Race | |||
| ŌĆāAsian | 25 (78%) | 5 (50%) | 28 (93%) |
| ŌĆāWhite | 6 (19%) | 4 (40%) | 1 (3%) |
| ŌĆāOther | 1 (3%) | 1 (10%) | 1 (3%) |
| Previous NUC treatment, months | 98.9 (53.8) | 0 (0) | 0.1 (0.4)* |
| ŌĆāMinŌĆōmax | 16.9ŌĆō213.9 | 0ŌĆō0 | 0ŌĆō2.0* |
| HBV DNA | |||
| ŌĆālog10 IU/mL | <LLOQ | 5.73 (1.86) | 6.91 (1.89) |
| ŌĆā>7 log10 IU/mL | 0 | 2 (20%) | 18 (60%) |
| HBV RNA | |||
| ŌĆāŌēźLLOQ | 15 (47%) | 8 (80%) | 27 (90%) |
| ŌĆālog10 copies/mLŌĆĪ | 2.58 (1.06) | 4.14 (1.41) | 5.1 (1.93) |
| HBsAg, | |||
| ŌĆālog10 IU/mL | 3.20 (0.52) | 3.48 (0.68) | 3.96 (0.9) |
| ŌĆāŌēź4 log10 IU/mL | 4 (13%) | 1 (10%) | 16 (53%) |
| HBeAg | |||
| ŌĆāPositiveŌĆĀ | 11 (34%) | 6 (60%) | 19 (63%) |
| ŌĆāNegative | 21 (66%) | 4 (40%) | 11 (37%) |
| ŌĆālog10 IU/mL┬¦ | 0.32 (0.77) | 1.06 (0.85) | 2.73 (0.60) |
| HBcrAg | |||
| ŌĆāŌēźLLOQ | 25 (78%) | 9 (90%) | 27 (90%) |
| ŌĆālog10 U/mLŌĆĪ | 4.68 (1.04) | 5.90 (1.67) | 7.29 (1.88) |
| HBV genotype | |||
| ŌĆāA | 1 (3%) | 0 | 1 (3%) |
| ŌĆāB | 8 (25%) | 0 | 13 (43%) |
| ŌĆāC | 6 (19%) | 5 (50%) | 11 (37%) |
| ŌĆāD | 0 | 5 (50%) | 2 (7%) |
| ŌĆāUnknown | 17 (53%) | 0 | 3 (10%) |
| ALT | |||
| ŌĆāU/L | 20.66 (7.30) | 59.40 (36.57) | 94.10 (42.96) |
| ŌĆāNormal | 32 (100%) | 1 (10%) | 2 (7%) |
| ŌĆā>1ŌĆō2xULN | 0 | 7 (70%) | 11 (37%) |
| ŌĆā>2ŌĆō5xULN | 0 | 2 (20%) | 17 (57%) |
Data are presented as mean (standard deviation) or number (%).
ALT, alanine aminotransferase; HBV, hepatitis B virus; HBcrAg, hepatitis core-related antigen; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; LLOQ, lower limit of quantification; NUC, nucleos(t)ide analogue; ULN, upper limit of normal.
* Only one patient received Lamivudine from Aug to Sep in 2011 (the exact start and end dates were unknown) before screening in 2020.
Table┬Ā2.
| HBsAg |
Linvencorvir+NUC+Peg-IFN-╬▒ (Cohort C) |
||
|---|---|---|---|
| Overall | HBeAg+ | HBeAg- | |
| Baseline | n=30 | n=19 | n=11 |
| ŌĆālog10 IU/mL | 3.96 (0.90) | 4.40 (0.71) | 3.19 (0.63) |
| ŌĆā<3 log10 IU/mL | 6 (20%) | 1 (5%) | 5 (45%) |
| ŌĆā<2 log10 IU/mL | 0 | 0 | 0 |
| At Week 48 | n=28 | n=18 | n=10 |
| ŌĆāCFB, log10 IU/mL | ŌĆō1.39 (0.98) | ŌĆō1.64 (0.90) | ŌĆō0.94 (0.99) |
| ŌĆāŌĆāGenotype B* | ŌĆō1.35 (0.62) | ŌĆō1.30 (0.64) | ŌĆō1.50 (0.62) |
| ŌĆāŌĆāGenotype CŌĆĀ | ŌĆō1.74 (1.13) | ŌĆō2.08 (1.05) | ŌĆō0.84 (0.91) |
| ŌĆāŌēź0.5 log10 IU/mL CFB | 21 (75.0%) | 16 (88.9%) | 5 (50.0%) |
| ŌĆā>1.0 log10 IU/mL CFB | 20 (71.4%) | 15 (83.3%) | 5 (50.0%) |
| ŌĆā>2.0 log10 IU/mL CFB | 7 (25.0%) | 5 (27.8%) | 2 (20.0%) |
| ŌĆā<3 log10 IU/mL | 19 (68%) | 12 (61%) | 8 (80%) |
| ŌĆā<2 log10 IU/mL | 6 (21%) | 2 (11%) | 4 (40%) |
Table┬Ā3.
|
Linvencorvir+NUC |
Linvencorvir+NUC+Peg-IFN-╬▒ Cohort C (n=30) | ||
|---|---|---|---|
| Cohort A (n=32) | Cohort B (n=10) | ||
| Patients with at least one AE, n (%) | 22 (69%) | 9 (90%) | 30 (100) |
| Total number of AEs | 110 | 48 | 468 |
| Total number of treatment-related AEs | 4 | 5 | 301 |
| ŌĆāLinvencorvir | 4 | 4 | 74 |
| ŌĆāNUC | 0 | 1 | 25 |
| ŌĆāPeg-IFN | NA | NA | 266 |
| Most common AEs*, n (%) | |||
| ŌĆāHeadache | 3 (9%) | 2 (20%) | 14 (47%) |
| ŌĆāPyrexia | 0 | 1 (10%) | 18 (60%) |
| ŌĆāALT increased | 1 (3%) | 1 (10%) | 11 (37%) |
| ŌĆāAlopecia | 0 | 1 (10%) | 11 (37%) |
| ŌĆāPlatelet count decreased | 0 | 0 | 12 (40%) |
| ŌĆāFatigue | 1 (3%) | 0 | 9 (30%) |
| ŌĆāAST elevation | 1 (3%) | 0 | 9 (30%) |
| ŌĆāDecreased appetite | 0 | 0 | 10 (33%) |
| Patients with at least one, n (%) | |||
| ŌĆāAE with fatal outcome | 0 | 1 (10%)ŌĆĪ | 0 |
| ŌĆāSAE | 1 (3%)ŌĆĀ | 1 (10%)ŌĆĪ | 2 (7%)┬¦ |
| ŌĆāAE leading to withdrawal | 0 | 0 | 2 (7%)┬Č |
| ŌĆāAE leading to Linvencorvir/Peg-IFN interruptionŌĆ¢ or modification | 1 (3%)/NA | 1 (10%)/NA | 5 (17%)/12 (40%) |
| ŌĆāRelated AE | 3 (9%) | 3 (30%) | 30 (100%) |
| ŌĆāŌĆāRelated to Linvencorvir | 3 (9%) | 2 (20%) | 21 (70%) |
| ŌĆāŌĆāRelated to NUC | 0 | 1 (10%) | 10 (33%) |
| ŌĆāŌĆāRelated to Peg-IFN | NA | NA | 30 (100%) |
| ŌĆāGrade 3ŌĆō4 AE | 4 (13%) | 2 (20%) | 11 (37%) |
| ŌĆāNon-ALT elevation-associated Grade 3ŌĆō4 AE | 3 (9%) | 1 (10%) | 9 (30%) |
AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NA, not applicable; NUC, nucleos(t)ide analogue; Peg-IFN, pegylated interferon; SAE, serious adverse event; ULN, upper limit of normal; URTI, upper respiratory tract infection.
ŌĆĪ The patient died on Day 535 due to malignant melanoma (SAE) onset on Day 425, with unresolved cellulitis and lymphadenitis (SAE diagnosed on Day 446).
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Wen Zhang

https://orcid.org/0000-0003-1939-4807Man-Fung Yuen

https://orcid.org/0000-0001-7985-7725 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print



