| Clin Mol Hepatol > Volume 29(Suppl); 2023 > Article |
|
ABSTRACT
Non-alcoholic fatty liver disease (NAFLD) is becoming the most common liver disease worldwide, and its burden is expected to increase due to the growing epidemic of obesity and diabetes. The key challenge among NAFLD patients is to identify those with advanced fibrosis (F3F4), who are at high risk of developing complications and will benefit from specialized management and treatment with new pharmacotherapies when they are approved. Liver biopsy appears unrealistic and unsuitable in practice, given the large number of high-risk patients and its well-known limitations. Non-invasive sequential algorithms using fibrosis-4 index as first-line test, followed by vibration-controlled transient elastography or patented blood test, are the best strategy for case finding of high-risk subjects. In fact, they are now recommended by several international guidelines, and should be used and disseminated to increase awareness among physicians beyond liver clinics where most NAFLD patients are seen.
Non-alcoholic fatty liver disease (NAFLD) affects around one-fourth of the general population worldwide [1]. NAFLD encompasses a wide range of lesions, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), with faster liver fibrosis progression as well as the risk of developing cirrhosis and its complications, including hepatocellular carcinoma (HCC) [2]. However, the vast majority of NAFLD patients will not progress, and only a minority, namely those with NASH and advanced fibrosis (F3, bridging fibrosis and F4, cirrhosis), are at the greatest risk of developing complications of chronic liver disease [3]. Patients with metabolic risk factors, particularly obesity and type 2 diabetes (T2DM), are at the highest risk of progressing to cirrhosis and HCC [4]. Due to the growing epidemic of obesity and diabetes, the burden of NAFLD is expected to increase [5]. Despite its high burden, NAFLD remains a largely under-recognized disease in primary care where most patients are seen. Additionally, the majority of NAFLD cases are asymptomatic with mild liver test abnormalities, making their identification a tough challenge for physicians in their daily clinical practice. As a result, less than 10% of patients diagnosed with NAFLD are referred to specialists [6]. Finally, there currently is no approved pharmacologic treatment for NAFLD. Therefore, the key challenge is to identify the minority of NAFLD patients with advanced fibrosis, who are at the greatest risk of developing complications, and will benefit from specialized management and treatment with new pharmacotherapies when they are approved.
For many years, liver biopsy has been considered the gold standard for the staging of liver fibrosis. However, it appears unrealistic and unsuitable, given the large number of highrisk patients and its well-known limitations [7]. Non-invasive strategies have been proposed as an alternative, and they have been an area of intensive research over the past decade [8]. These strategies include serum biomarkers of fibrosis and liver stiffness measurement (LSM), using either ultrasound- or magnetic resonance-based elastography techniques. Although none of these non-invasive tests can adequately discriminate NASH from simple steatosis in patients with NAFLD, they are now extensively used in liver clinics to detect advanced liver fibrosis and are recommended by international guidelines [9-11]. In this review, we discuss the performance, advantages, and limitations of non-invasive tests for identifying high-risk NAFLD patients.
In NAFLD patients, NASH is the driver of fibrosis progression, but the presence of NASH without significant fibrosis is not associated with increased liver-related mortality or overall mortality [12,13]. probably due to the competing mortality risks of cardiovascular disease and non-liver related cancers in these patients. Several studies have reported that, besides the high rate of liver-related complication, the risk of allcause mortality is clearly increased in NAFLD patients with advanced fibrosis [14,15]. In addition, two meta-analyses, based mostly on longitudinal retrospective studies, have shown that, the main prognosis driver for liver-related and overall mortality in NAFLD patients is the stage of liver fibrosis, namely advanced fibrosis [16,17]. These findings have been recently confirmed prospectively in the NASH CRN cohort (n=1,773 NAFLD patients), followed over a median period of 4.0 years (total: 8,120 person years) [18]. Indeed, all-cause mortality increased with increasing fibrosis stages (0.32 deaths per 100 person-years for stage F0 to F2, 0.89 deaths per 100 persons-years for stage F3, and 1.76 deaths per 100 personyears for stage F4. Thus, it has now been well established that the risk of liver-related complications in NAFLD exponentially increases when transitioning to stage F3 and then F4. Advanced fibrosis is, therefore, the primary lesion to target when designing strategies to detect high-risk NAFLD patients who have the worse clinical outcomes.
Despite major efforts in the development of new drugs [19]. no pharmacologic treatment has yet been approved for NAFLD. The current consensus is that pharmacotherapy should be reserved for patients with NASH and at least significant fibrosis. At-risk NASH (or fibrotic NASH) is defined by the presence of NASH (NAFLD activity score Ōēź4 with one item of each, at least) and significant fibrosis (fibrosis stage Ōēź2) [20]. Identification of these patients is important in tertiary referral centers, as they are the main target population for ongoing NASH phase 2 and 3 trials.
The most commonly used non-patented blood tests are the fibrosis-4 index (FIB-4) and the NAFLD fibrosis score (NFS). The FIB-4 includes four simple parameters: age, platelets, and serum transaminases (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]). The NFS includes seven parameters: age, body mass index (BMI), impaired fasting glucose/T2DM, AST, ALT, platelets, and albumin. The FIB-4 was initially developed for the non-invasive diagnosis of fibrosis in a set of 555 patients with HIV-chronic hepatitis C co-infection [21], and then was also evaluated for the diagnosis of advanced liver fibrosis in NAFLD [22]. Contrary to FIB-4, the NFS has been developed specifically in NAFLD, in a large set including 480 patients [23]. Evidence for the accuracy of FIB-4 and NFS in NAFLD has now reached the level of meta-analysis, with area under the receiver-operating-characteristic curve (AUROC) at 0.76 for FIB-4 and 0.73 for NFS [22]. The results of these two tests were interpreted using two diagnostic thresholds, a lower to rule-out advanced liver fibrosis (FIB-4 <1.30, NFS <-1.455), and a higher to rule-in advanced liver fibrosis (FIB-4 >2.67, NFS >0.676). Meta-analyses have shown that the sensitivity for advanced liver fibrosis with the rule-out threshold of FIB-4 and NFS is acceptable, at around 75%, and specificity with the rule-in threshold is very good at 94% [22,24].
Context of use, particularly in a clinical setting, is important when dealing with blood tests, knowing that NFS is not the best test for the screening of advanced liver fibrosis in patients with T2DM [25-27]. Also, age [28] and BMI [29], included in the NFS formula, affect its performance in older patients with morbid obesity. By contrast, FIB-4, which is only affected by age, seems to be a better option in these populations. Both FIB-4 and NFS can be calculated for free through websites and smartphone applications. FIB-4, however, is the most popular and most studied non-patented blood fibrosis test due to its simplicity and the fact that serum transaminases and platelet count are largely prescribed by general practitioners (GPs) in their check-up for metabolic diseases. In large populations of unselected patients, at a threshold of 1.30, FIB-4 has the strong advantage to very easily rule-out a large proportion (60ŌĆō80%) of the subjects evaluated [30]. Moreover, repeating FIB-4 measurement could evaluate the risk of liverrelated complication [31] within time.
The most studied patented blood fibrosis tests in NAFLD include FibroTest®, FibroMeterTM, and Enhanced Liver Fibrosis (ELFTM) test [7]. These non-invasive tests combine indirect and direct markers of liver fibrosis, the latter being components of liver fibrosis or proteins directly involved in the processes of fibrogenesis and fibrolysis in the liver during chronic liver diseases. Recent meta-analyses evaluating the accuracy of these tests in NAFLD patients reported an AUROC for advanced liver fibrosis of 0.77 for FibroTest® [32], 0.83 for ELFTM [33]. and 0.89 for FibroMeterTM [34]. Direct comparison performed in 417 patients with biopsy-proven NAFLD has found similar diagnostic accuracy between FibroMeterTM and ELFTM. 35 Patented blood tests are more accurate than non-patented blood tests [35,36], but their cost and limited availability limit their widespread application. Therefore, they are more suited when used as a second-line option, to further confirm the risk of advanced liver fibrosis suggested by the first-line non-patented blood fibrosis test.
Importantly, studies are concordant about the fact that negative predictive values (NPVs) for excluding advanced fibrosis are higher than the corresponding positive predictive values (PPVs). Thus, blood tests may be confidently used for first-line risk stratification to exclude advanced fibrosis. However, most of these studies have been conducted in tertiary referral centers where the pre-test probability of advanced fibrosis is higher (20ŌĆō30%) than that in primary care (<5%), which could have a major impact in the accuracy results [37].
Elastography include ultrasound-based techniques, such as vibration-controlled transient elastography (VCTE) (FibroScan, Echosens, France), point shear wave elastography (pSWE), two-dimensional shear wave elastography (2D-SWE), and magnetic resonance elastography (MRE) [38]. Among them, VCTE is the method with the largest amount of evidence [7]. Two large multicenter studies [39,40] reported high VCTE applicability (96ŌĆō97%) in NAFLD patients. Moreover, the same cutoffs can be used without further adjustment for steatosis when the M and XL probes are used according to the appropriate BMI (30 kg/m2). In a recent meta-analysis including 5,489 NAFLD patients in 37 studies, VCTE had excellent accuracy for diagnosing advanced fibrosis and cirrhosis, with AUROCs of 0.85 and 0.90, respectively [22].
As for the remaining techniques, a recent systematic review of 82 studies (14,609 patients) and a meta-analysis of 70 studies (12,547 patients) showed that only MRE and pSWE had a specificity greater than 80% for the diagnosis of advanced fibrosis (89% and 86%, respectively) [41]. Nonetheless, all evaluated techniques had a good diagnostic accuracy. The reported summary AUROC for diagnosing advanced fibrosis with VCTE, MRE, pSWE, and 2D-SWE were 0.85, 0.92, 0.89, and 0.72, respectively [41]. Although MRE had the best diagnostic accuracy, it remains a research tool due to its limited availability and cost. Moreover, pSWE/ARFI and 2D-SWE are not included in the current guidelines on the management of NAFLD due to the limited amount of data [9-11]. Taken together, these results suggest that VCTE is currently the technique with the highest level of evidence to confidently exclude advanced fibrosis and cirrhosis with a high negative predictive value (around 90%) in NAFLD patients [7]. For example, VCTE had a 94% to 100% NPV at a cut-off <8 kPa. On the other hand, the PPV did not exceed 64% at a cut-off >10 kPa. Finally, VCTE is a point-of care technique, available in most liver clinics worldwide, and is thus the technique of choice for the second-line testing of advanced fibrosis.
The context of use is critical when using non-invasive tests, as it will strongly influence their diagnostic performance. The pretest probability of the target condition (advanced fibrosis) will impact PPV and NPV [9]. When dealing with patients in primary care, where the prevalence of advanced fibrosis is low (<5%), non-invasive tests are far better for ruling out (high NPV) rather than for diagnosing (high PPV) the presence of advanced fibrosis. This indicates the need for at least two tiers of non-invasive fibrosis tests for selecting patients from low-prevalence populations for further investigations and follow-up to reduce false positive results [42]. Therefore, using widely available, easy-to-obtain, and cheap blood tests (nonpatented serum markers) as the first-line procedure followed, if positive, by a second-line confirmatory test (elastography or patented serum markers) seems the most appropriate strategy. The use of sequential algorithms is more effective than single tests in both low and high prevalence settings [22,43].
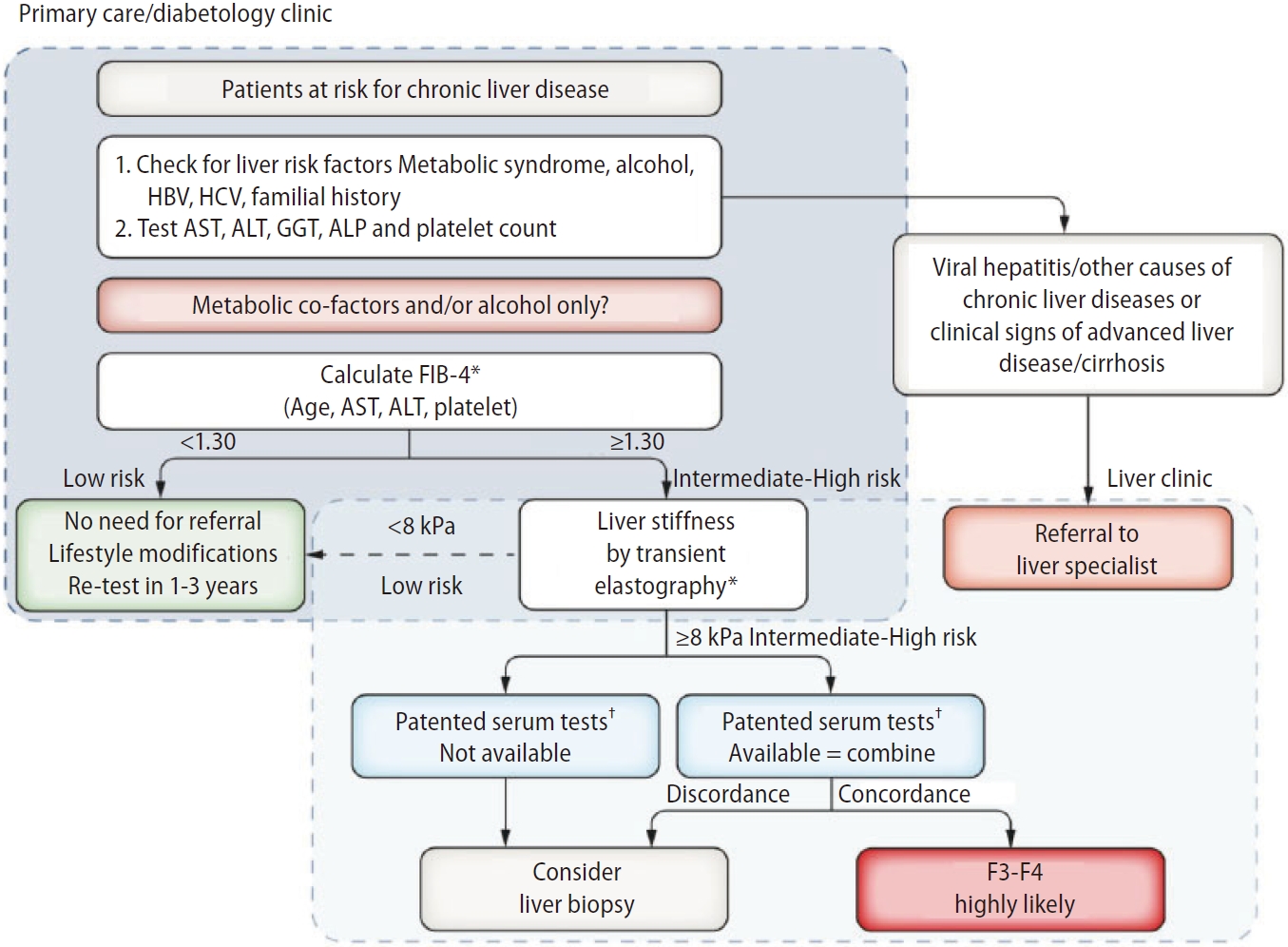
Several sequential strategies using non-invasive tests have been proposed to identify patients with advanced fibrosis in clinical practice [9-11]. The first algorithm proposed by the European Association for the Study of Liver (EASL) targets patients with high risk of NAFLD seen in primary care or diabetology clinics, using FIB-4 (single cutoff 1.3) followed by VCTE (single cutoff 8.0 kPa) (Fig. 1) [9]. Patients with FIB-4 >1.3 are considered to be at intermediate-high risk of advanced fibrosis and should undergo VCTE, which may be performed before or after referral to liver specialist according to local availability and pathways. Finally, in patients with LSM >8.0 kPa, a third test (a patented blood test) can be performed, if available. In case of concordant results with VCTE, advanced fibrosis is highly likely. Otherwise, liver biopsy may be considered when results are discordant results or if a patented blood test is unavailable. Patients with FIB-4 <1.3 and/or LSM <8.0 kPa have a low risk of advanced fibrosis and can be monitored by their GP with repeated measurements during follow-up. The use of this algorithm in ŌĆ£real lifeŌĆØ has been recently validated in a retrospective, multicenter French cohort of 1,051 patients with biopsy-proven NAFLD [44]. Compared with the performance of single non-invasive tests (NITs), agreement between two NITs (FIB4 and VCTE, VCTE and patented serum tests) increased specificity and PPV by 20%, thereby justifying the sequential use proposed in the EASL algorithm. The FIB-4/VCTE/FibroMeterTM and FIB-4/VCTE/FibroTest┬« algorithms performed similarly, providing 85% diagnostic accuracy and a liver biopsy requirement rate of only 10%.
Interestingly, in the same cohort of patients, the EASL algorithm was also able to predict the risk of liver-related events (LRE) [45]. In patients with FIB-4 >1.3, the risk of LRE increased according to the VCTE results with adjusted hazard ratio of 3.9 (95% confidence interval [95% CI] 1.3ŌĆō10.9) in those with 8.0<LSM<12.0 kPa and 12.4 (95% CI 5.1ŌĆō30.2) in those with LSM >12.0 kPa. Finally, the utility of EASL algorithm has been examined in 467 patients with type 2 diabetes seen in primary care, independently from their transaminase levels [46]. Twenty of 440 (4.5%) patients were found to have advanced liver disease, compared to three patients who were previously identified through standard care (odds ratio 6.71, 95% CI 2.0ŌĆō22.7; P=0.002). Alcohol and obesity were predictors of advanced disease, a finding consistent with previous studies [47-49].
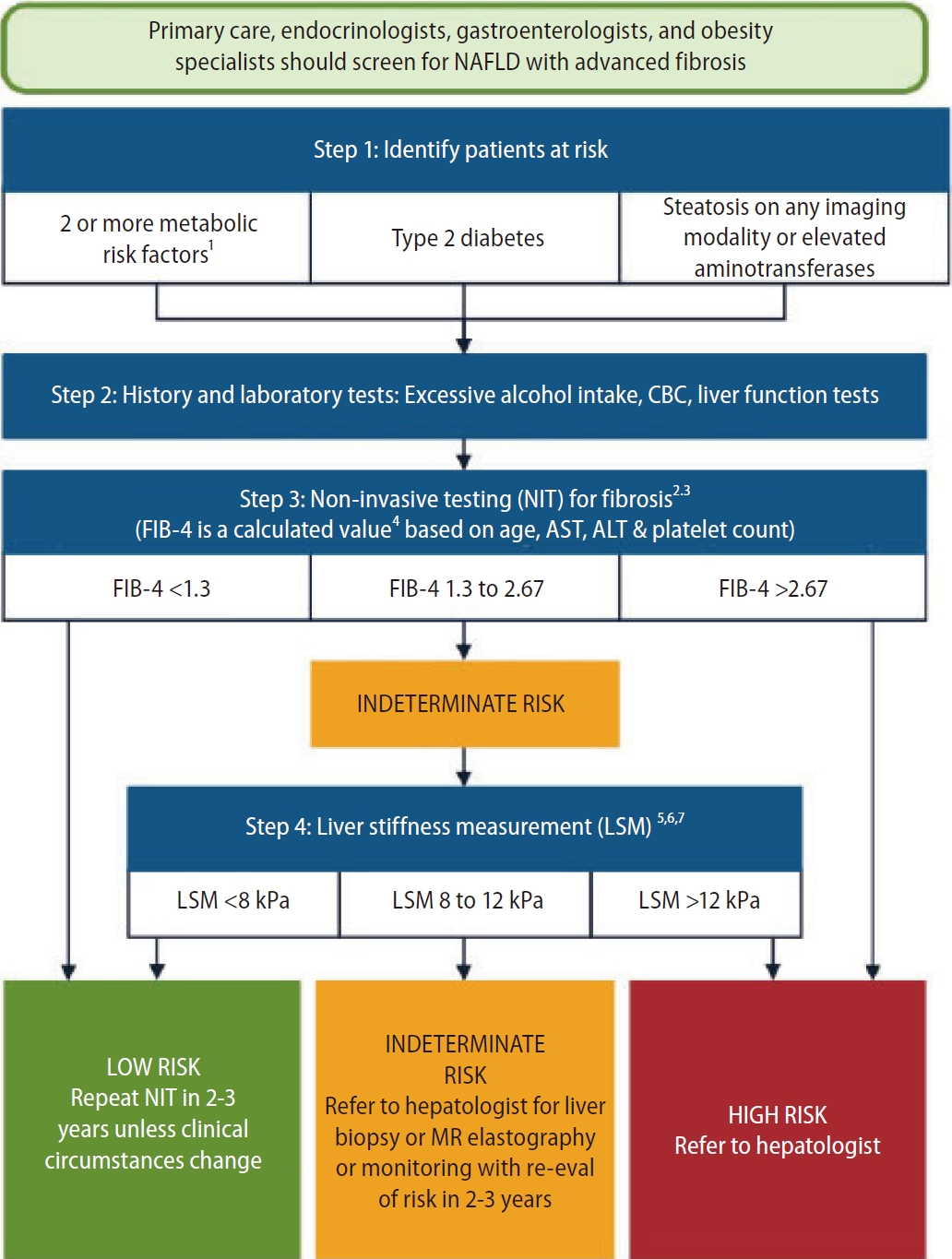
Other algorithms have been proposed since, including the American Gastroenterology Association (AGA) pathway [11] and the American Association of Clinical Endocrinology (AACE) algorithm [10]. The AGA pathway targets the same population as the EASL algorithm, and uses FIB-4 (dual cutoffs 1.3ŌĆō2.67) followed by VCTE (dual cutoffs 8.0ŌĆō12.0 kPa) (Fig. 2). Patients with FIB-4 in between 1.3 and 2.67 are considered as indeterminate risk, and should undergo VCTE. Patients with FIB-4 <1.3 and/or LSM <8.0 kPa are considered to be at low risk of advanced fibrosis, and can be monitored by their GP with repeated measurements during follow-up. Those with 8.0<LSM<12.0 kPa are considered at indeterminate risk, and should be referred to an hepatologist for liver biopsy or MRE. Those with FIB-4 >2.67 or LSM >12 kPa are considered at high risk, and should be referred to an hepatologist.
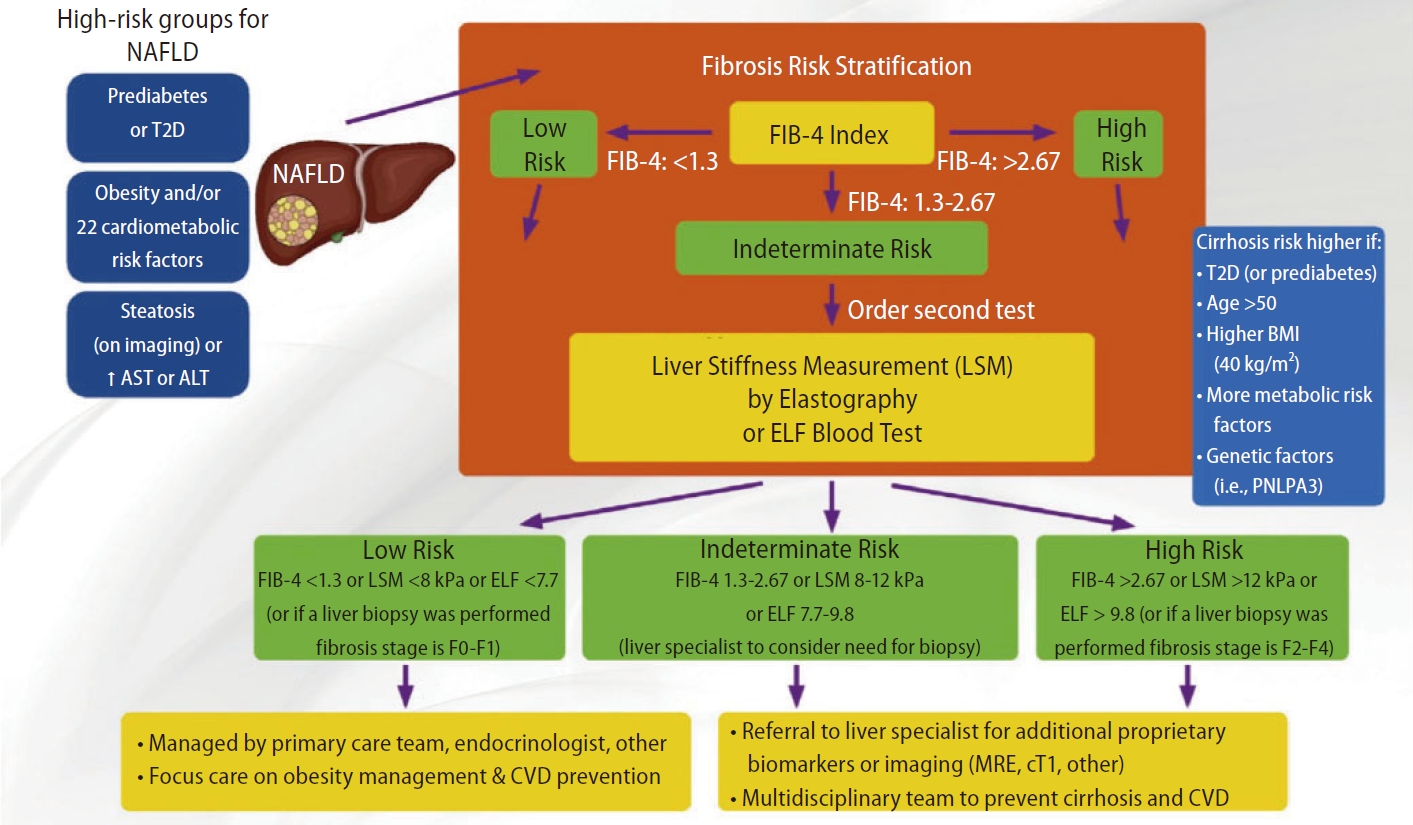
As for the AACE algorithm, it is very similar to the AGA pathway but consider the use of ELFTM (dual cutoffs 7.7ŌĆō9.8) as an alternativeto VCTE in patients with FIB-4 in between 1.3 and 2.67 (Fig. 3). Patients with indeterminate risk (FIB-4 1.3ŌĆō2.67 or LSM 8ŌĆō12 kPa or ELFTM 7.7ŌĆō9.8) and high risk (FIB-4 >2.67 or LSM >12 kPa or ELFTM >9.8) should be referred to an hepatologist for liver biopsy or MRE.
In summary, it is noteworthy that over the past year, guidelines from Hepatology, Gastroenterology, and Endocrinology International Societies recommended very similar sequential non-invasive strategies using the same tools and cutoffs. This will likely simplify the case finding and management of high-risk patients with NAFLD in clinical practice.
Several non-invasive scores combining serum and imaging biomarkers have been proposed to diagnose at-risk NASH patients. The first score is the FibroScan-AST (FAST), a continuous and composite score, combining controlled attenuation parameter (CAP), LSM by VCTE, and AST level [50]. The score ranges from 0 to 1 with a 0.35 rule-out cutoff (Ōēź90% sensitivity) and a 0.67 rule-in cutoff (Ōēź90% specificity). Patients with values between the two cutoffs are in the grey zone with indeterminate results. FAST had an AUROC of 0.85 for the detection of at-risk NASH patients in the pooled external validation cohort, with NPV of 94% for ruling out and PPV of 69% for ruling in at-risk NASH, respectively (Table 1). Overall, 60% of patients could be correctly classified and avoid a liver biopsy. It should be acknowledged that performances of FAST may vary according to the prevalence of at-risk NASH patients in the applied population. For instance, in a USA cohort with a 12% prevalence, FAST had an AUROC of 0.86, allowing to classify 78% of patients, whereas in another cohort from Turkey with a 57% prevalence, its AUROC was 0.74, with 43% of patients correctly classified [50].
The second score, the magnetic resonance imaging (MRI)-AST (MAST) score, is based on the FAST concept, but using MRI (PDFF and MRE) instead of VCTE [51]. MAST had an AUROC of 0.93, a NPV of 98% for ruling out and a PPV of 50% for ruling in at-risk NASH, respectively (Table 1). Overall, 70% of patients could be correctly classified and avoid a liver biopsy. Finally, the MRE combined with FIB-4 (MEFIB) index, a categorical score combining MRE and FIB-4, has been proposed, but with a different primary objective of detecting F2-4 fibrosis in NAFLD [52]. When evaluated for at-risk NASH, MEFIB had an AUROC of 0.77, a NPV of 93% for ruling out and a PPV of 55% for ruling in at-risk NASH, respectively (Table 1). Overall, 57% of patients could be correctly classified.
Head-to-head comparison of FAST, MAST, and MEFIB showed conflicting results. One study suggested that MAST outperformed FAST [51], whereas another study suggested that MEFIB outperformed both MAST and FAST [53]. These results deserve several comments. First, one of the challenges with these scores was dealing with patients in the grey zone. Interestingly, in the study comparing the three scores [53], the grey zone of MAST was significantly smaller than that of FAST and MEFIB (8.5% vs. 40.1% and 24.7%, respectively; P<0.001). As a result, the number of patients correctly classified as at-risk NASH was higher with MAST than with MEFIB and FAST (69.4% vs. 57.4% and 45.3%, respectively) [54]. Second, when compared independently from the developers in a large cohort of T2DM patients with NAFLD, MAST and FAST outperformed MEFIB, and MAST allowed to correctly classify the largest number of patients [55]. However, high cost and limited availability may compromise widespread application of MRI-based scores. Further studies are needed.
The high-risk population in NAFLD patients is now wellidentified (i.e., patients with advanced fibrosis), and simple non-invasive tools are available for case finding. Algorithms based on these non-invasive tools are effective and recommended by several international guidelines, but are mostly validated thus far in tertiary referral liver centers. The next step is to implement these algorithms beyond the liver clinics, particularly in primary care and diabetes clinics where most NAFLD patients are seen. The main barrier against is the lack of awareness among physicians managing these patients. Indeed, NAFLD remains largely unknown outside the fields of hepatology and gastroenterology, and is overlooked by most physicians [56]. As a result, less than 10% of NAFLD patients are referred to a specialist and opportunities for early interventions are missed, particularly in those with advanced fibrosis [6]. In addition, NAFLD is absent from nearly all national and international strategies and policies for non-communicable diseases, including obesity [57]. Therefore, dissemination of guidelines on the use of non-invasive tests and multidisciplinary approaches are critical to increase awareness and to improve management of NAFLD patients.
Figure┬Ā1.
EASL algorithm. FIB-4 can be used in patients with metabolic co-factors and/or alcoholic liver disease to identify patients requiring referral to the liver clinic (FIB-4 >1.3). VCTE may be performed before or after referral to liver specialist according to local availability and pathways. Adapted from the article of EASL (J Hepatol 2021;75:659-689) [9]. EASL, European Association for the Study of Liver; FIB-4, fibrosis-4; VCTE, vibration controlled transient elastography. *Transient elastography or FIB-4 may be performed before or after referral to liver specialist according to local availability and pathways. ŌĆĀCut-offs to use: ELFTM 9.8 (NAFLD/ALD); FibroMeter 0.45 (NAFLD), Fibrotest 0.48 (NAFLD).
Figure┬Ā2.
AGA pathway. FIB-4 (dual cutoffs 1.3ŌĆō2.67) is used as first-line followed by VCTE (dual cutoffs 8.0ŌĆō12.0 kPa). Adapted from the article of Kanwal et al. (Gastroenterology 2021;161:1657-1669) [11]. AGA, American Gastroenterology Association; FIB-4, fibrosis-4; VCTE, vibration controlled transient elastography; NAFLD, non-alcoholic fatty liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; MR, magnetic resonance.
Figure┬Ā3.
AACE algorithm. FIB-4 (dual cutoffs 1.3ŌĆō2.67) is used as first-line followed by VCTE (dual cutoffs 8.0ŌĆō12.0 kPa). ELFTM (dual cutoffs 7.7ŌĆō9.8) can be used as an alternative to VCTE in patients with FIB-4 in between 1.3 and 2.67. Adapted from the article of Cusi et al. (Endocr Pract 2022;28:528-562) [10]. AACE, American Association of Clinical Endocrinology; FIB-4, fibrosis-4; VCTE, vibration controlled transient elastography; ELFTM, Enhanced Liver Fibrosis; NAFLD, non-alcoholic fatty liver disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T2D, type 2 diabetes; BMI, body mass index; MRE, magnetic resonance elastography; CVD, cardiovascular disease.
Table┬Ā1.
Diagnostic performances of FAST, MAST, and MEFIB scores for the diagnosis of ŌĆ£at-riskŌĆØ NASH patients
NASH, non-alcoholic steatohepatitis; AUROC, area under the ROC curve; Se, sensitivity; Sp, specificity; NPV, negative predictive value; PPV, positive predictive value; AST, aspartate aminotransferase; FAST, FibroScan-AST; MRI, magnetic resonance imaging; MAST, MRI-AST; MRE, magnetic resonance elastography; FIB-4, fibrosis-4; MEFIB, MRE combined with FIB-4.
Correctly classified (CC)=true negative for rule-out cutoff+true positive for rule-in cutoff/total.
REFERENCES
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84.


3. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643-654.e1-9 quiz e39-40.



4. Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 2020;71:808-819.



5. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018;69:896-904.


6. Blais P, Husain N, Kramer JR, Kowalkowski M, El-Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol 2015;110:10-14.



7. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1264-1281 e4.



8. Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: Past, present and future. J Hepatol 2022;76:1362-1378.


9. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol 2021;75:659-689.


10. Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract 2022;28:528-562.

11. Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657-1669.



12. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013;145:782-789.e4.



13. Hagstr├Čm H, Nasr P, Ekstedt M, Hammar U, St├źl P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265-1273.


14. Ekstedt M, Hagstr├Čm H, Nasr P, Fredrikson M, St├źl P, Kechagias S, et al. Fibrosis stage is the strongest predictor for diseasespecific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547-1554.


15. Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: Interprotocol agreement and ability to predict liverrelated mortality. Hepatology 2011;53:1874-1882.



16. Taylor RS, Taylor RJ, Bayliss S, Hagstr├Čm H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology 2020;158:1611-1625 e12.


17. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557-1565.




18. Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al.; NASH Clinical Research Network (CRN). Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559-1569.



19. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al.; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394:2184-2196 Erratum in: Lancet 2020;396:312, Lancet 2021;397:2336.

20. Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L; American Association for the Study of Liver Diseases; United States Food and Drug Administration. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: Findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology 2015;61:1392-1405.



21. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al.; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-1325.


22. M├│zes FE, Lee JA, Selvaraj EA, Jayaswal ANA, Trauner M, Boursier J, et al.; LITMUS Investigators. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006-1019.



23. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846-854.


24. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017;66:1486-1501.



25. Alkayyali T, Qutranji L, Kaya E, Bakir A, Yilmaz Y. Clinical utility of noninvasive scores in assessing advanced hepatic fibrosis in patients with type 2 diabetes mellitus: a study in biopsy-proven non-alcoholic fatty liver disease. Acta Diabetol 2020;57:613-618.



26. Boursier J, Canivet CM, Costentin C, Lannes A, Delamarre A, Sturm N, et al. Impact of type 2 diabetes on the accuracy of noninvasive tests of liver fibrosis with resulting clinical implications. Clin Gastroenterol Hepatol 2022 Mar 11;doi: 10.1016/j.cgh.2022.02.059.


27. Patel P, Hossain F, Horsfall LU, Banh X, Hayward KL, Williams S, et al. A pragmatic approach identifies a high rate of nonalcoholic fatty liver disease with advanced fibrosis in diabetes clinics and at-risk populations in primary care. Hepatol Commun 2018;2:893-905.




28. McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a confounding factor for the accurate noninvasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol 2017;112:740-751.



29. Qadri S, Ahlholm N, L├Ėnsmann I, Pellegrini P, Poikola A, Luukkonen PK, et al. Obesity modifies the performance of fibrosis biomarkers in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2022;107:e2008-e2020.




30. Unalp-Arida A, Ruhl CE. Noninvasive fatty liver markers predict liver disease mortality in the U.S. population. Hepatology 2016;63:1170-1183.




31. Hagstr├Čm H, Talb├żck M, Andreasson A, Walldius G, Hammar N. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol 2020;73:1023-1029.


32. Vali Y, Lee J, Boursier J, Spijker R, Verheij J, Brosnan MJ, et al.; On Behalf Of The Litmus Systematic Review Team. FibroTest for evaluating fibrosis in non-alcoholic fatty liver disease patients: A systematic review and meta-analysis. J Clin Med 2021;10:2415.



33. Vali Y, Lee J, Boursier J, Spijker R, Verheij J, Brosnan MJ, et al.; On Behalf Of The Litmus Systematic Review Team. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J Hepatol 2020;73:252-262.


34. Van Dijk AM, Vali Y, Mak AL, Lee J, Tushuizen ME, Zafarmand MH, et al. Systematic review with meta-analyses: Diagnostic accuracy of FibroMeter tests in patients with non-alcoholic fatty liver disease. J Clin Med 2021;10:2910.



35. Guillaume M, Moal V, Delabaudiere C, Zuberbuhler F, Robic MA, Lannes A, et al. Direct comparison of the specialised blood fibrosis tests FibroMeterV2G and Enhanced Liver Fibrosis score in patients with non-alcoholic fatty liver disease from tertiary care centres. Aliment Pharmacol Ther 2019;50:1214-1222.



36. Boursier J, Guillaume M, Leroy V, Irl├©s M, Roux M, Lannes A, et al. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J Hepatol 2019;71:389-396.


37. Castera L, Boursier J. Noninvasive algorithms for the case finding of ŌĆ£At-RiskŌĆØ patients with NAFLD. Semin Liver Dis 2022;42:313-326.


38. Friedrich-Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol 2016;13:402-411.



39. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717-1730.


40. Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, et al.; NASH Clinical Research Network. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:156-163.e2 .



41. Selvaraj EA, M├│zes FE, Jayaswal ANA, Zafarmand MH, Vali Y, Lee JA, et al.; LITMUS Investigators. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J Hepatol 2021;75:770-785.

42. Tsochatzis EA, Newsome PN. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol 2018;3:509-517.


43. Majumdar A, Campos S, Gurusamy K, Pinzani M, Tsochatzis EA. Defining the minimum acceptable diagnostic accuracy of noninvasive fibrosis testing in cirrhosis: A decision analytic modeling study. Hepatology 2020;71:627-642.



44. Canivet CM, Costentin C, Irvine KM, Delamarre A, Lannes A, Sturm N, et al. Validation of the new 2021 EASL algorithm for the noninvasive diagnosis of advanced fibrosis in NAFLD. Hepatology 2022 Jul 13;doi: 10.1002/hep.32665.



45. Boursier J, Hagstr├Čm H, Ekstedt M, Moreau C, Bonacci M, Cure S, et al. Non-invasive tests accurately stratify patients with NAFLD based on their risk of liver-related events. J Hepatol 2022;76:1013-1020.


46. Mansour D, Grapes A, Herscovitz M, Cassidy P, Vernazza J, Broad A, et al. Embedding assessment of liver fibrosis into routine diabetic review in primary care. JHEP Rep 2021;3:100293.



47. Glyn-Owen K, B├Čhning D, Parkes J, Roderick P, Buchanan R. The combined effect of alcohol and body mass index on risk of chronic liver disease: A systematic review and meta-analysis of cohort studies. Liver Int 2021;41:1216-1226.



48. Harman DJ, Ryder SD, James MW, Wilkes EA, Card TR, Aithal GP, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: A cross-sectional study using transient elastography. Aliment Pharmacol Ther 2018;47:504-515.



49. Innes H, Crooks CJ, Aspinall E, Card TR, Hamill V, Dillon J, et al. Characterizing the risk interplay between alcohol intake and body mass index on cirrhosis morbidity. Hepatology 2022;75:369-378.



50. Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:362-373 Erratum in: Lancet Gastroenterol Hepatol 2020;5:e3.



51. Noureddin M, Truong E, Gornbein JA, Saouaf R, Guindi M, Todo T, et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J Hepatol 2022;76:781-787.


52. Jung J, Loomba RR, Imajo K, Madamba E, Gandhi S, Bettencourt R, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2021;70:1946-1953.



53. Kim BK, Tamaki N, Imajo K, Yoneda M, Sutter N, Jung J, et al. Head-to-head comparison between MEFIB, MAST, and FAST for detecting stage 2 fibrosis or higher among patients with NAFLD. J Hepatol 2022;77:1482-1490.


54. Noureddin M, Harrison SA, Alkhouri N. MEFIB vs MAST and FAST: Not a competition but useful tools. J Hepatol 2022 Nov 9;doi: 10.1016/j.jhep.2022.10.020.


55. Castera L, Garteiser P, Laou├®nan C, Vallet-Pichard A, Vidal-Trecan T, Manchon P, et al. Prospective head-to-head comparison of FAST, MAST, MEFIB, NFS and FIB-4 scores for diagnosing fibrotic NASH in patients with type 2 diabetes. Hepatology 2022;76 Suppl:S86 Abstract no. 97.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





