Dear Editor,
Polycystic liver disease is a genetically heterogeneous disorder, involving derangements on at least three different chromosomes [
1,
2]. Some patients with polycystic liver disease may develop complications as the result of massive hepatomegaly or progress to advanced liver disease. In the symptomatic polycystic liver disease patient, surgical therapy and/or liver transplantation remain the mainstay of therapy [
2,
3]. Although the benefits of tolvaptan, an inhibitor of vasopressin type 2 (V2) receptor, on the progression of renal dysfunction in autosomal dominant polycystic kidney disease (ADPKD) are well established [
4], the influences on liver cysts have not been well delineated. Indeed, only one report was seen until now [
5]. We recently experienced a case suggesting the efficacy of tolvaptan to manage polycystic liver disease. The present case indicated that tolvaptan reduced liver as well as kidney volume in ADPKD, presumably by shrinking cysts.
A 37-year-old woman with family history of ADPKD complained abdominal fullness.
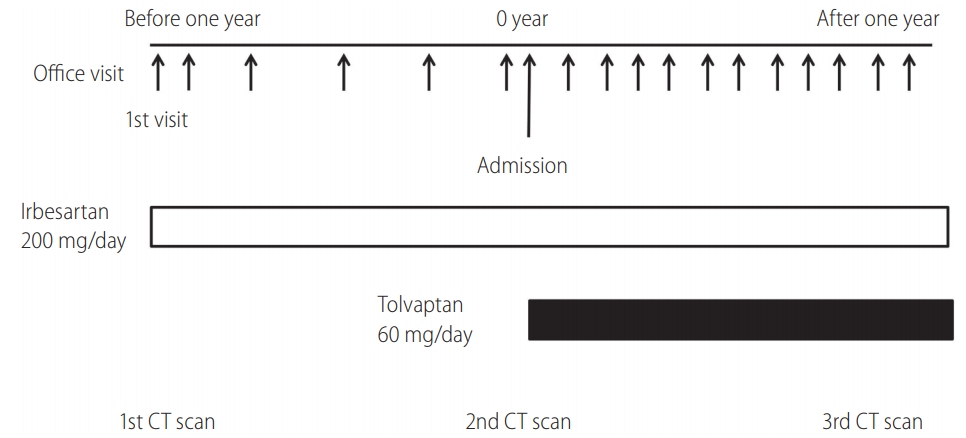
Figure 1 showed her clinical course. Physical examination blood pressure of 144/102 mmHg and hepatomegaly, the liver and kidney function were normal (
Table 1, prothrombin time-international normalized ratio: 1.02). Abdominal computed tomography (CT) showed polycystic liver and kidneys (
Fig. 2). The liver and the total kidney volume were 8,674 and 1,024 mL, respectively (
Fig. 3). The CT scan data were transferred to the workstation (Ziostation system 610, Amin Co., Ltd., Tokyo, Japan) to generate 3D image and assess organ volume. Left and right kidney volumes were combined to calculate the total kidney volume. Since she preferred medical management, irbesartan (200 mg/day) was first prescribed after counselling on family planning [
6]. A year later, her blood pressure dropped to 115/84 mmHg. Although her liver volume was essentially unchanged to 8,781 mL, kidney volume was increased to 1,194 mL.
She started to take tolvaptan (60 mg/day) to limit the growth of renal cysts [
4]. Of note, Japanese Ministry of Health, Labour and Welfare approved to apply large doses of tolvaptan (up to 120 mg/day) for the patients with progressive ADPKD. Since then, she has been visiting our office once a month (
Fig. 1). No adverse reactions including hepatic events were found at any visits (
Table 1). Another year later, her kidney and liver volume were reduced to 1,047 and 7,846 mL (
Fig. 3), suggesting that cysts in both kidneys and liver were shrunk. Notably, her abdominal fullness was improved, and abdominal CT revealed near-complete disappearance of a large hepatic cyst (74 mm in diameter) in the S6 segment (
Fig. 2). She neither complained abdominal pain or discomfort, nor exhibited abnormal liver function test at any visits, implicating that hepatic cyst rupture was unlikely. She is still taking irbesartan (200 mg/day) and tolvaptan (60 mg/day) without any side effects.
Recent studies show that cholangiocyte autophagy, which is associated with activation of the cyclic adenosine monophosphate (cAMP)-protein kinase A and of cAMP response element-binding protein signaling pathway, contributes to cystogenesis in polycystic liver disease [
7]. Further, follicle-stimulating hormone (FSH) receptors are seen in biliary epithelial cells from normal and ADPKD patients [
8]. FSH increases c-AMP in cholangiocytes, inducing biliary growth via ERK. In addition, Mancinelli et al. have demonstrated that cholangiocytes express V2 receptors that are upregulated in the liver of ADPKD patients, and that vasopressin causes an increase in the proliferation and cAMP, a second messenger of V2 receptor, in human cholangiocytes from the cystic epithelium [
9]. Indeed, tolvaptan inhibits the vasopressin-induced increase in cAMP in cholangiocytes.
Clinically, somatostatin analogues can be used for medical management of polycystic liver disease [
10]. Lanreotide (120 mg) reduced liver volume by 3% in 6 months. Somatostatin analogues are now mainly used for endocrine diseases as they exhibit severe adverse effects such as gallstone. An aberrant relation between intracellular calcium and c-AMP is proposed as the mechanism of liver cyst formation [
3,
7]. As discussed, tolvaptan reduces c-AMP in cholangiocytes [
9]. Although 14 out of 135 ADPKD patients experienced hepatic events in Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) extension Japan trial, they recovered after the interruption of tolvaptan [
11,
12].
Collectively, our case is consistent with the notion that tolvaptan suppresses liver cyst growth by reducing cAMP to inhibit autophagy and proliferation of cholangiocytes in polycystic liver disease [
7,
9], and suggests that tolvaptan treatment can be performed safely in patients with polycystic liver disease. These provide potential applicability of tolvaptan for polycystic liver disease patients with normal liver function to manage hepatomegaly, and warrant further clinical investigations to examine the effects of tolvaptan as a novel agent militating against liver cyst progression [
3].
ACKNOWLEDGMENTS
The patient gave us an informed consent for this publication.
Figure┬Ā1.
Timeline of the case. We asked the patient to admit when starting tolvaptan to circumvent severe adverse reactions such as dehydration and liver dysfunction, and advised her to take the amount of fluid similar to urine volume. The administration of tolvaptan increased urine volume from 1,250 to 3,700 mL/day. CT, computed tomography
Figure┬Ā2.
Abdominal computed tomography scan was obtained at her first visit (before one year), prior to administration of tolvaptan (0 year) and a year after tolvaptan treatment (after one year), in a patient with polycystic liver disease. The scan shows disappearance of the previously visualised liver cyst in the S6 segment. R, right; L, left.

Figure┬Ā3.
Time course of the liver and the total kidney volume. Tolvaptan was started at 0 year. Tolvaptan treatment for a year induced approximately 10% reductions in liver (A) and kidney (B) volume.

Table┬Ā1.
Time course of blood biochemical profile
|
Time (months)
|
|
Before 12 |
Before 6 |
0 |
3 |
6 |
9 |
12 |
|
Alb (g/dL) |
4.6 |
4.5 |
4.6 |
4.5 |
4.6 |
4.8 |
4.5 |
|
TB (mg/dL) |
0.6 |
0.9 |
0.8 |
0.7 |
0.9 |
1.0 |
0.7 |
|
LDL-C (mg/dL) |
88 |
109 |
108 |
103 |
91 |
107 |
104 |
|
HDL-C (mg/dL) |
56 |
66 |
65 |
69 |
60 |
71 |
69 |
|
AST (U/L) |
14 |
20 |
28 |
19 |
21 |
23 |
19 |
|
ALT (U/L) |
12 |
15 |
24 |
14 |
15 |
22 |
13 |
|
GGT (U/L) |
28 |
27 |
36 |
36 |
38 |
40 |
37 |
|
Cr (mg/dL) |
0.76 |
0.74 |
0.72 |
0.72 |
0.73 |
0.73 |
0.71 |
|
Na (mEq/L) |
140 |
142 |
140 |
139 |
139 |
139 |
140 |
Abbreviations
ADPKD
autosomal dominant polycystic kidney disease
cAMP
cyclic adenosine monophosphate
FSH
follicle-stimulating hormone
TEMPO
Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes
REFERENCES
1. Nakanuma Y, Hoso M, Hayashi M, Hirai N. Adult polycystic liver presenting with progressive hepatic failure. J Clin Gastroenterol 1989;11:592-594.


2. Arnold HL, Harrison SA. New advances in evaluation and management of patients with polycystic liver disease. Am J Gastroenterol 2005;100:2569-2582.


10. van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2009;137:1661-1668 e1-e2.










 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



