| Clin Mol Hepatol > Volume 22(2); 2016 > Article |
|
ABSTRACT
Iodine-131 is a radioisotope that is routinely used for the treatment of differentiated thyroid cancer after total or near-total thyroidectomy. However, there is some evidence that iodine-131 can induce liver injury . Here we report a rare case of drug-induced liver injury (DILI) caused by iodine-131 in a patient with regional lymph node metastasis after total thyroidectomy. A 47-year-old woman was admitted with elevated liver enzymes and symptoms of general weakness and nausea. Ten weeks earlier she had undergone a total thyroidectomy for papillary thyroid carcinoma and had subsequently been prescribed levothyroxine to reduce the level of thyroid-stimulating hormone. Eight weeks after surgery she underwent iodine-131 ablative therapy at a dose of 100 millicuries, and subsequently presented with acute hepatitis after 10 days. To rule out all possible causative factors, abdominal ultrasonography, endoscopic ultrasonography (on the biliary tree and gall bladder), and a liver biopsy were performed. DILI caused by iodine-131 was suspected. Oral prednisolone was started at 30 mg/day, to which the patient responded well.
The incidence of differentiated thyroid cancer (DTC) has increased globally over the last several decades, and iodine-131 is routinely used to treat DTC after total or near-total thyroidectomy to ablate the remnant thyroid tissue and enable physicians to detect tumor recurrence or distant metastasis more easily [1]. Iodine-131 is also used to treat benign thyroid conditions such as Grave’s disease and benign nodular goiter [2].
Conventional doses of iodine-131 are 75–100 millicurie (mCi; 2,775–3,700 millibecquerel) [3]. Few case reports have found that iodine-131 induced liver injury, including a case report involving a patient with Grave’s disease [4,5]. Here we present a case of drug-induced liver injury (DILI) that occurred 10 days after iodine-131 ablative therapy and responded favorably to oral corticosteroid.
A 47-year-old woman was referred to our hepatology unit in May 2015 for the evaluation of increased liver enzymes. The patient was previously diagnosed with thyroid cancer (T3N1aM0) in March 2015 and had undergone total thyroidectomy with right neck lymph node dissection. At 8 weeks after surgery, she underwent iodine-131 therapy at 100 mCi; 10 days later, she was admitted with jaundice and elevated liver enzymes.
The patient was taking only prescribed medications, including levothyroxine 150 µg, calcitriol 0.25 µg, and calcium carbonate 500 mg daily for 2 months after the total thyroidectomy. She denied having any alcohol or smoking history and taking any possible agents causing hepatitis such as health supplements or herbal medications.
On admission, her vital signs were normal, and a physical examination showed no remarkable findings except icteric sclerae and skin. Mild hepatomegaly was observed; however, there was no evidence of splenomegaly or ascites. Her blood count revealed a white blood cell count of 3,550/L (neutrophils, 35.9%; lymphocytes, 49.9%; and eosinophils 3.2%), and hemoglobin level, hematocrit level, and platelet count were 13.4 g/dL, 38.9%, and 148,000/L, respectively. Serum biochemical assay gave the following results: blood urea nitrogen (BUN) level, 8.0 mg/dL; creatinine level, 0.85 mg/dL; sodium level, 142 mEq/L; potassium level, 3.9 mEq/L; albumin level, 4.3 g/dL; aspartate aminotransferase (AST) level, 1,136 IU/L; alanine transaminase (ALT) level, 1,632 IU/L; total bilirubin level, 2.0 mg/dL; direct bilirubin level, 1.3 mg/dL; γ-glutamyl transpeptidase (γ-GT) level, 303 IU/L; alkaline phosphatase (ALP) level, 713 IU/L; and lactate dehydrogenase (LDH) level, 502 IU/L.
Serological tests for immunoglobulin M (IgM) anti-hepatitis A virus, hepatitis B surface antigen, IgM anti-hepatitis B core antibody, anti-hepatitis C virus (HCV) antibody, HCV real-time polymerase chain reaction, anti-hepatitis E virus antibody, human immunodeficiency virus (HIV) antigen, anti-HIV antibody, IgM anti-cytomegalovirus, IgM anti-Epstein-Barr virus viral-capsid antigen, and IgM anti-herpes simplex virus were negative, as were results for autoimmune markers, including anti-nuclear antibody, anti-mitochondrial Ab, anti-smooth muscle Ab, and anti-liver kidney microsome antibody-1. Serum thyroid stimulation hormone level was 23.64 mIU/L (0.17–5.65 mIU/L), serum free T4 level was 1.46 ng/dL (0.80–1.90 ng/dL), and serum T3 level was 124.9 ng/dL (78.0–182.0 ng/dL).
Abdominal ultrasonography revealed prominent periportal interstitial echogenicity, suggesting hepatitis (Fig. 1). A liver biopsy was performed to confirm the diagnosis, and a histological examination revealed that the lesion was composed of moderate lobular inflammation and mild portal inflammation without fibrosis (Fig. 2). The Roussel Uclaf Causality Assessment Method (RUCAM) score was 6, which was consistent with probable DILI (Table 1). In the analysis of R value, our case was 2.3, suggestive of mixed-type DILI.
Viral and autoimmune hepatitis were excluded based on the above findings. A diagnosis of DILI caused by iodine-131 was made as the administration of levothyroxine, calcitriol, and calcium carbonate for 2 months did not raise any special issues.
A blood test conducted on the third day of hospitalization showed that AST/ALT was 1,308/1,920 IU/L, total bilirubin was 2.9 mg/dL, and ALP was 675 IU/L, indicating the aggravation of jaundice. Since her symptoms of nausea, vomiting, and abdominal pain persisted, administration of daily 30 mg oral prednisolone was initiated. Subsequently, her clinical indicators improved and her AST/ALT, total bilirubin, and ALP levels started to decrease. After 10 days on prednisolone, the patient was discharged with considerably improved AST/ALT and total bilirubin levels (180/174 IU/L and 0.9 mg/dL; Fig. 3).
Iodine-131 ablation therapy after the surgical procedure of total or near-total thyroidectomy is standard management for thyroid cancer [1]. Iodine-131 is generally considered able to ablate thyroid cells without affecting healthy tissues in the body because of their levothyroxine (T4) and triiodothyronine (T3) contents. However, iodine-131 can accumulate in other body tissues and destroy normal tissues because iodine is distributed through the bloodstream and can be accumulated in any other organs associated with thyroid hormone metabolism [6]. According to previous published reports, diffuse iodine-131 uptake in the liver was revealed frequently in the post-therapy scan, and the uptake amount increases in the status of total thyroidectomy [7-9]. Therefore, the status of thyroidectomy might be associated with an increased risk of iodine-131-related liver injury; however, the detailed mechanism involved is not well understood. Few previous studies have reported on DILI after iodine-131 therapy. Lin et al. [4] reported hepatotoxicity after iodine-131 therapy in a patient with thyroid cancer who subsequently responded to methylprednisolone. Another report described two cases of liver toxicity after iodine-131 ablation therapy in two patients with Grave’s disease, both of whom were treated with prednisolone [5].
In our case, several factors are able to potentiate the diagnosis of iodine-131-induced hepatotoxicity, including female sex, timing of drug intake and withdrawal, clinical course of liver injury, and exclusion of other causes of liver injury [10]. When the RUCAM scale was applied to our patient, the assessed score was consistent with probable DILI (Table 1) [11]. Thus, we concluded that liver injury in this case was probably caused by iodine-131 ablative therapy.
Since most cases of DILI follow a benign course, the main treatment for DILI is withdrawal of the causative agent with careful observation for risk of acute liver failure [12]. Majority of the patients with DILI experience complete recovery after discontinuing the offending drug. However, 13–17% of acute liver failure cases have been attributed to idiosyncratic drug reactions, with a high mortality rate (80%) without liver transplantation [13,14]. Elevated liver enzyme (ALT > 3 times, bilirubin > 2 times the upper limit of normal) is considered a predictive factor of severe DILI. In such cases, a mortality of at least 10% can be expected [15].
In our patient’s clinical course, the liver enzymes were markedly increased (AST/ALT was 1,308/1,920 IU/L, total bilirubin was 2.9 mg/dL) and gastrointestinal symptoms (abdominal pain, nausea, and vomiting) were aggravated after hospitalization despite the provision of supportive treatment. A skin rash also developed on her trunk and limbs. These findings indicated rapid progression of DILI, for which we decided to start oral corticosteroid (prednisolone 30 mg per day) to relieve the symptoms and achieve the expected therapeutic effect.
Steroid therapy is generally used to recover the features of autoimmune hepatitis (AIH) in DILI and reduce the allergic symptoms in other types of DILI [16]. Since the mechanism of DILI after iodine-131 ablation was not established, whether steroid therapy might be effective remains unclear. Nevertheless we could give consideration that it might be partly induced by a hypersensitivity reaction to the iodine-131 in the same way as AIH. In fact, it is difficult to distinguish AIH from DILI, because overall histological and serological features of these diseases are not far different from each other. And the frequent appearance of autoimmune hepatitis at the second episode of DILI, named ‘drug-induced autoimmune hepatitis (DI-AIH)’, makes it more challenging [17]. The immune-mediated reactions is the leading mechanism of DI-AIH, but it has no pathognomonic features revealed and specific diagnostic criteria confirmed until now [18].
Previous studies reported that patients with DILI after iodine-131 ablation responded dramatically to steroid therapy. These results can be supported by our finding that our patient also responded favorably to steroid therapy; however, further studies are needed to support the actual therapeutic efficacy of this agent.
The incidence of thyroid cancer continues to increase, and iodine-131 ablative therapy is routinely considered after total thyroidectomy. However, it is important to consider the possibility of DILI by monitoring the liver enzymes. More evidence is required to determine whether iodine-131 is related to the incidence of hepatitis and whether some patients are more vulnerable than others.
Figure 1.
Abdominal ultrasonograph showing a prominent periportal interstitial echogenicity, suggesting hepatitis.
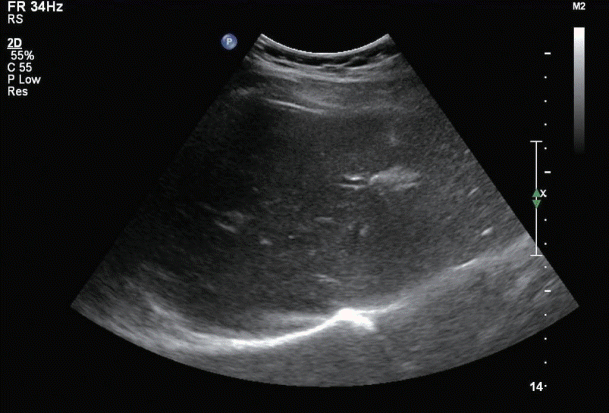
Figure 2.
Photomicrograph of a liver biopsy sample showing moderate lobular inflammation and mild portal inflammation without fibrosis, suggesting acute hepatitis (H & E stain, ×400).
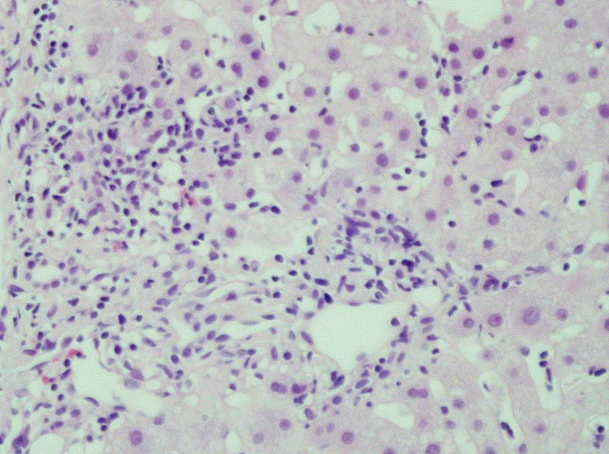
Figure 3.
After starting oral prednisolone at 30 mg/day, the serum levels of aspartate aminotransferase (AST), alanine transaminase (ALT), and total bilirubin began to decrease.
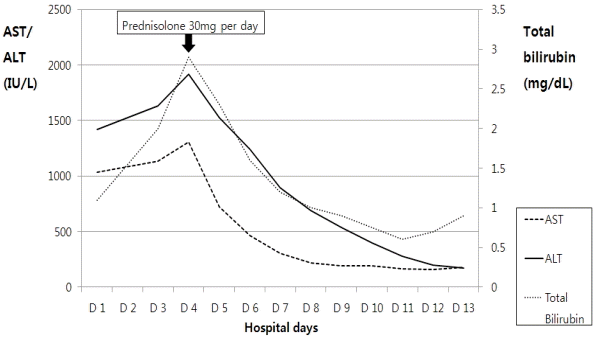
Table 1.
Patient’s score on the Roussel Uclaf Causality Assessment Method scale.
REFERENCES
1. Mayson SE, Yoo DC, Gopalakrishnan G. The evolving use of radioiodine therapy in differentiated thyroid cancer. Oncology 2015;88:247-256.


2. Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev 2012;33:920-980.


3. Doi SA, Woodhouse NJ, Thalib L, Onitilo A. Ablation of the thyroid remnant and I-131 dose in differentiated thyroid cancer: a meta-analysis revisited. Clin Med Res 2007;5:87-90.



4. Lin R, Banafea O, Ye J. I-131 remnant ablation after thyroidectomy induced hepatotoxicity in a case of thyroid cancer. BMC Gastroenterol 2015;15:56.




5. Jhummon NP, Tohooloo B, Qu S. Iodine-131 induced hepatotoxicity in previously healthy patients with Grave’s disease. Thyroid Res 2013;6:4.



6. Changlai SP, Chang PJ, Chen CY. Biodistribution and dosimetry of (131) I in thyroidectomy patients using semiquantitative gammacamera imaging. Cancer Biother Radiopharm 2008;23:759-766.


7. Ferris HA, Williams G, Parker JA, Garber JR. Therapeutic implications of diffuse hepatic uptake following I-131 therapy for differentiated thyroid cancer. Endocr Pract 2013;19:263-267.


8. Tatar FA, Morita E, Ituarte PH, Cavalieri RR, Duh QY, Price DC, et al. Association between residual thyroid carcinoma and diffuse hepatic uptake of 131I following radioiodine ablation in postoperative total thyroidectomy patients. World J Surg 2001;25:718-722.


9. Rosenbaum RC, Johnston GS, Valente WA. Frequency of hepatic visualization during I-131 imaging for metastatic thyroid carcinoma. Clin Nucl Med 1988;13:657-660.


10. Heidari R, Niknahad H, Jamshidzadeh A, Abdoli N. Factors affecting drug-induced liver injury: antithyroid drugs as instances. Clin Mol Hepatol 2014;20:237-248.



11. Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323-1330.


13. Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2000;137:947-954.

14. Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005;40:1095-1101.


16. Maddur H, Chalasani N. Idiosyncratic drug-induced liver injury: a clinical update. Curr Gastroenterol Rep 2011;13:65-71.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




