| Clin Mol Hepatol > Volume 27(4); 2021 > Article |
|
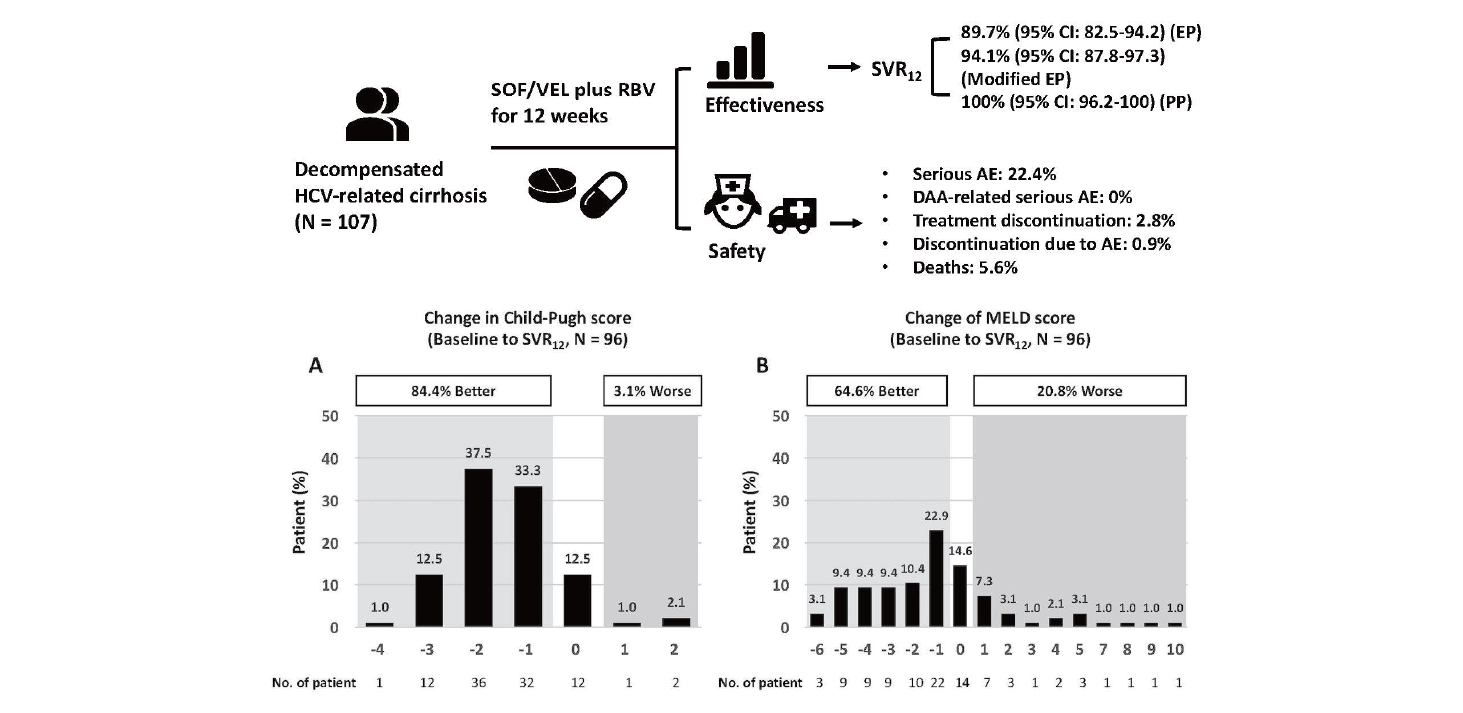
ABSTRACT
Background/Aims
Methods
Results
ACKNOWLEDGMENTS
FOOTNOTES
SUPPLEMENTAL MATERIAL
Supplementary┬ĀFigure┬Ā1.
Figure┬Ā1.
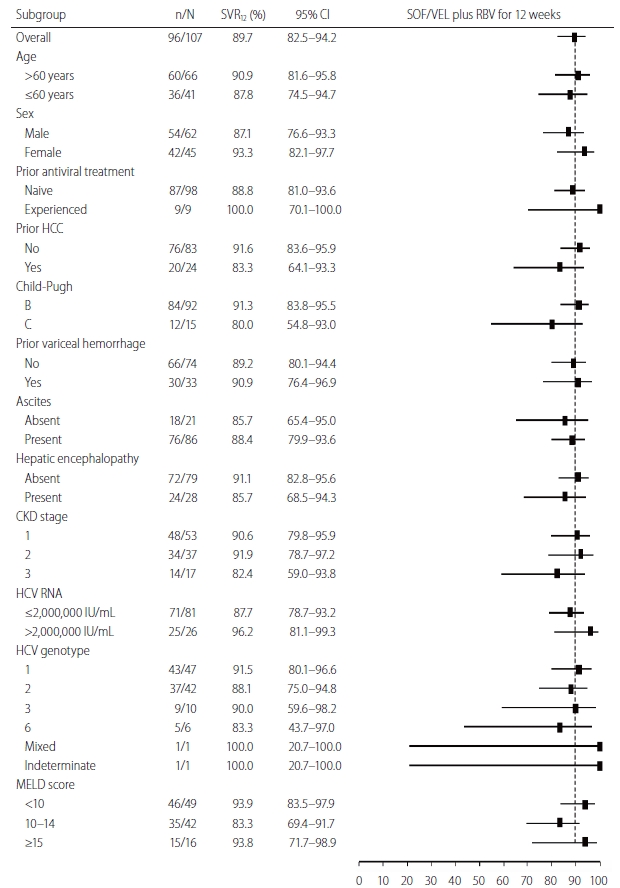
Figure┬Ā2.
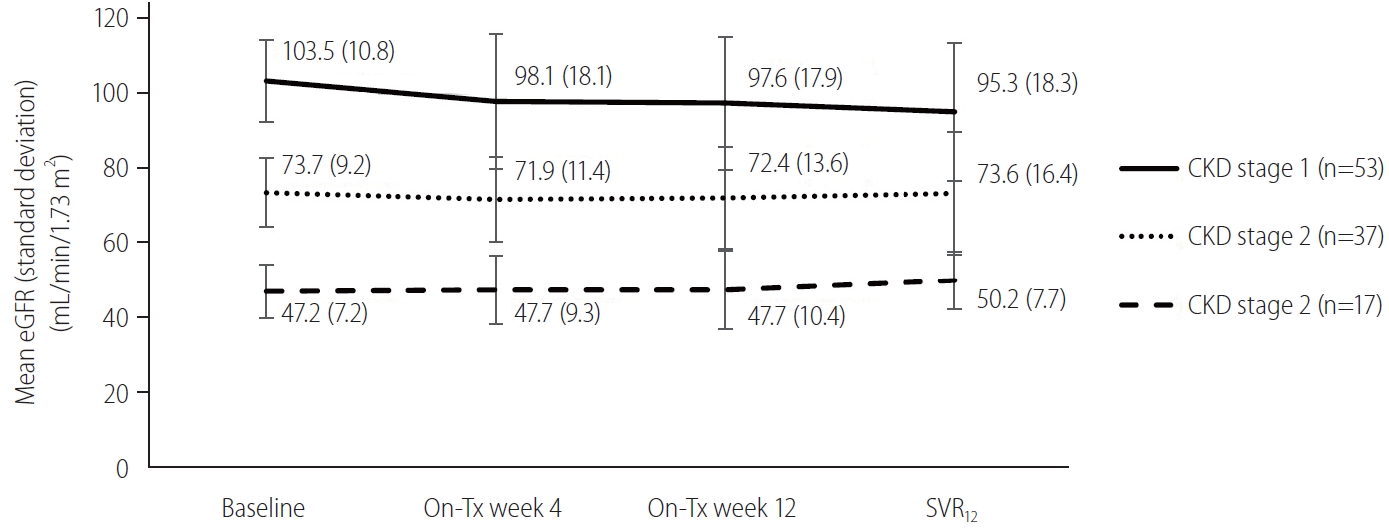
Figure┬Ā3.
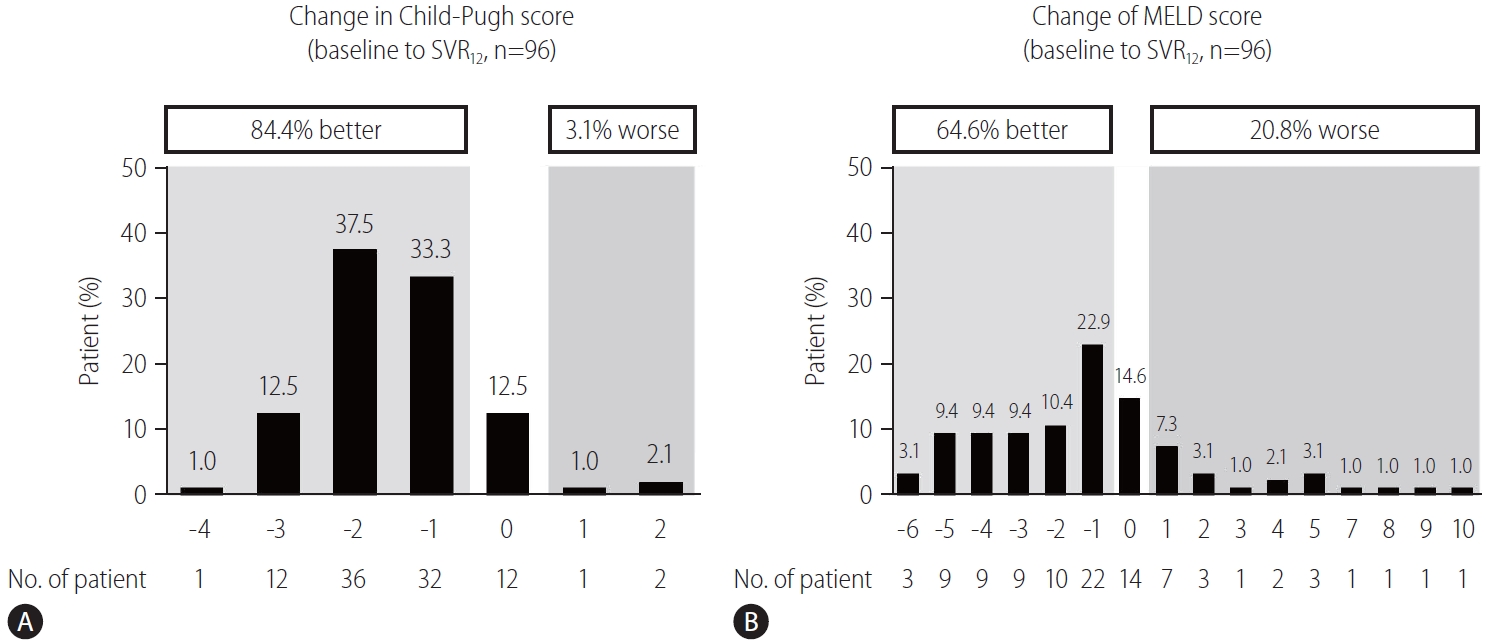
Figure┬Ā4.
Table┬Ā1.
| Characteristic | SOF/VEL plus RBV (n=107) |
|---|---|
| Age (years) | 65 (56ŌĆō73) |
| Age >60 years | 66 (61.7) |
| Male | 62 (57.9) |
| Prior antiviral treatment | |
| ŌĆāNa├»ve | 98 (91.6) |
| ŌĆāExperienced | 9 (8.4) |
| ŌĆāPR | 9 (100.0) |
| Grade of hepatic decompensation | |
| ŌĆāChild-Pugh B | 92 (86.0) |
| ŌĆāChild-Pugh C | 15 (14.0) |
| History of HCC | |
| ŌĆāNo | 83 (77.6) |
| ŌĆāYes | 24 (22.4) |
| Prior variceal hemorrhage | |
| ŌĆāNo | 74 (69.2) |
| ŌĆāYes | 33 (30.8) |
| Ascites | |
| ŌĆāNone | 21 (19.6) |
| ŌĆāMild to moderate | 82 (76.6) |
| ŌĆāSevere | 4 (3.7) |
| Hepatic encephalopathy | |
| ŌĆāNone | 79 (73.8) |
| ŌĆāMild to moderate | 28 (26.2) |
| ŌĆāSevere | 0 (0.0) |
| HCV RNA (log10 IU/mL) | 5.6 (4.6ŌĆō6.3) |
| HCV RNA >2,000,000 IU/mL | 26 (24.3) |
| HCV genotype | |
| ŌĆā1 | 2 (1.9) |
| ŌĆā1a | 11 (10.3) |
| ŌĆā1b | 34 (31.8) |
| ŌĆā2 | 42 (39.3) |
| ŌĆā3 | 10 (9.3) |
| ŌĆā6 | 6 (5.6) |
| ŌĆāMixed | 1 (0.9) |
| ŌĆāIndeterminate | 1 (0.9) |
| Hemoglobin (g/dL) | 11.5 (10.7ŌĆō12.8) |
| WBC count (109 cells/L) | 4.4 (3.3ŌĆō6.1) |
| Platelet count (109 cells/L) | 86 (59ŌĆō116) |
| INR | 1.24 (1.11ŌĆō1.34) |
| Albumin (g/dL) | 2.9 (2.6ŌĆō3.3) |
| Total bilirubin (ULN)* | 2.2 (1.7ŌĆō3.1) |
| ALT (ULN)* | 2.2 (1.2ŌĆō3.3) |
| Creatinine (mg/dL) | 0.80 (0.70ŌĆō1.00) |
| eGFR (mL/min/1.73 m2)ŌĆĀ | 89 (67ŌĆō100) |
| eGFR (mL/min/1.73 m2) | |
| ŌĆāŌēź 90 (CKD stage 1) | 53 (49.5) |
| ŌĆā60ŌĆō89 (CKD stage 2) | 37 (34.6) |
| ŌĆā30ŌĆō59 (CKD stage 3) | 17 (15.9) |
| MELD score | 10 (7ŌĆō13) |
| MELD score | |
| ŌĆā<10 | 49 (45.8) |
| ŌĆā10ŌĆō14 | 42 (39.3) |
| ŌĆāŌēź15 | 16 (15.0) |
Values are presented as median (interquartile range) or number (%).
SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; PR, peginterferon plus ribavirin; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; WBC, white blood cell; INR, international normalized ratio; ULN, upper limit of normal; ALT, alanine transaminase; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; MELD, model for end-stage liver disease.
Table┬Ā2.
| HCV RNA < LLOQ* |
SOF/VEL plus RBV (n=107) |
|
|---|---|---|
| n/N (%) | 95% CI | |
| During treatment | ||
| ŌĆāWeek 12ŌĆĀ | 102/102 (100.0) | 96.4ŌĆō100.0 |
| After treatment | ||
| ŌĆāSVR12, EPŌĆĪ | 96/107 (89.7) | 82.5ŌĆō94.2 |
| ŌĆāSVR12, modified EP┬¦ | 96/102 (94.1) | 87.8ŌĆō97.3 |
| ŌĆāSVR12, PPŌłź | 96/96 (100.0) | 96.2ŌĆō100.0 |
| Patients not achieving SVR12 | ||
| ŌĆāOn-treatment | ||
| ŌĆāŌĆāDeath | 2 | |
| ŌĆāŌĆāPremature discontinuation | 3 | |
| ŌĆāOff-treatment | ||
| ŌĆāŌĆāDeath | 4 | |
| ŌĆāŌĆāLost to follow-up | 2 | |
| ŌĆāŌĆāRelapse | 0 | |
HCV, hepatitis C virus; LLOQ, lower limit of quantification; SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; CI, confidence interval; SVR12, sustained virologic response rate at off-treatment week 12; EP, evaluable population; PP, per-protocol population.
ŌĆĀ Two patients who died and three patients prematurely discontinued treatment before on-treatment week 12 did not have available data for the analysis.
Table┬Ā3.
| Event | SOF/VEL plus RBV (n=107) |
|---|---|
| Any AE | 98 (91.6) |
| Serious AE* | 24 (22.4) |
| DAA-related serious AE | 0 (0.0) |
| RBV-related serious AE | 0 (0.0) |
| Treatment discontinuationŌĆĀ | 3 (2.8) |
| Discontinuation due to treatment-emergent AEŌĆĪ | 1 (0.9) |
| Death┬¦ | 6 (5.6) |
| AE occurring in Ōēź10% of patients | |
| ŌĆāFatigue | 88 (82.2) |
| ŌĆāNausea | 24 (22.4) |
| ŌĆāHeadache | 21 (19.6) |
| ŌĆāInsomnia | 18 (16.8) |
| ŌĆāDiarrhea | 16 (15.0) |
| ŌĆāDizziness | 15 (14.0) |
| ŌĆāPruritus | 13 (12.1) |
| ŌĆāDyspnea | 11 (10.3) |
| Laboratory abnormalities | |
| ŌĆāHemoglobin | |
| ŌĆāŌĆāGrade 2 (8.0ŌĆō9.9 g/dL) | 28 (26.2) |
| ŌĆāŌĆāGrade 3 (<8.0 g/dL) | 6 (5.6) |
| ŌĆāWhite blood cell count | |
| ŌĆāŌĆāGrade 3 (1.0ŌĆō2.0├Ś109 cells/L) | 5 (4.7) |
| ŌĆāŌĆāGrade 4 (<1.0├Ś109 cells/L) | 1 (0.9) |
| ŌĆāPlatelet count | |
| ŌĆāŌĆāGrade 3 (25ŌĆō49├Ś109 cells/L) | 14 (13.1) |
| ŌĆāŌĆāGrade 4 (<25├Ś109 cells/L) | 0 (0.0) |
| ŌĆāTotal bilirubin | |
| ŌĆāŌĆāGrade 3 (3.0ŌĆō10.0├ŚULN)Ōłź | 31 (30.0) |
| ŌĆāŌĆāGrade 4 (>10.0├ŚULN) | 0 (0.0) |
| ŌĆāALT | |
| ŌĆāŌĆāGrade 3 (5.0ŌĆō20.0├ŚULN) | 0 (0.0) |
| ŌĆāŌĆāGrade 4 (>20.0├ŚULN) | 0 (0.0) |
Values are presented as number (%).
SOF, sofosbuvir; VEL, velpatasvir; RBV, ribavirin; AE, adverse event; DAA, direct-acting antiviral; ULN, upper limit of normal; ALT, alanine transaminase.
* Esophageal variceal hemorrhage (n=2), gastric variceal hemorrhage (n=2), spontaneous bacterial peritonitis (n=3), hepatic encephalopathy (n=4), hepatocellular carcinoma (n=4), pneumonia (n=3), urinary tract infection (n=2), ischemic bowel disease (n=1), femoral fracture with septic shock (n=1), lumbar epidural abscess (n=1), and traumatic head injury (n=1).
ŌĆĀ Fatigue (n=1), traumatic head injury (n=1), and lost to follow-up (n=1) at on-treatment weeks 2, 3, and 8, respectively.
┬¦ Gastric variceal hemorrhage at on-treatment week 1 (n=1), ischemic bowel disease at on-treatment week 11 (n=1), hepatic encephalopathy at off-treatment week 2 (n=1), femoral fracture with septic shock at off-treatment week 4 (n=1), pneumonia at off-treatment week 8 (n=1), and lumbar epidural abscess at off-treatment week 8 (n=1).
Table┬Ā4.
Abbreviations
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Jia-Horng Kao

https://orcid.org/0000-0002-2442-7952 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print



