| Korean J Hepatol > Volume 17(3); 2011 > Article |
ABSTRACT
Background/Aims
Pegylated interferon (peginterferon) and ribavirin combination therapy is less effective and associated with a higher frequency of serious complications in chronic hepatitis C patients with cirrhosis than in noncirrhotic patients. This study evaluated the efficacy and tolerability of peginterferon and ribavirin treatment in patients with hepatitis C virus (HCV)-related cirrhosis.
Methods
Eighty-six patients with clinically diagnosed liver cirrhosis were treated with either peginterferon alpha-2a (n=51) or peginterferon alpha-2b (n=35) plus ribavirin. The sustained virologic response (SVR) and adverse effects were analyzed retrospectively.
Results
Of the 86 patients (55 males), 48 patients (55.8%) had HCV genotype 1 infection and 38 (44.2%) had genotype non-1 infection. The overall SVR rate was 34.9% (30/86), and the rates of SVR in the genotype 1 and non-1 patients were 20.8% (10/48) and 52.6% (20/38), respectively. The multivariate analysis revealed that having HCV genotype 1 (P=0.003) and high baseline viral load (>8.0├Ś105 IU/mL, P=0.012) were the independent predictive factors for SVR failure. In 20.9% (18/86) of the patients, treatment was not completed due to adverse events (27.8%), loss to follow-up (50.0%), and other reasons (22.2%).
Hepatitis C virus (HCV) infection may affect 2.5-3.0% of the world's population.1 Among Koreans older than 40 years, 1.3% are positive for HCV antibodies.2 Liver cirrhosis may develop in 20% of those infected and 1-4% of the patients with cirrhosis may develop hepatocellular carcinoma (HCC) annually.3 Combination therapy with pegylated-interferon (peginterferon) and ribavirin reduces the rate of progression of liver fibrosis in patients with chronic hepatitis C.4 Interferon treatment for patients with HCV-related liver cirrhosis inhibits the development of HCC and it improves survival.5 These results indicate that cirrhotic patients with chronic hepatitis C as well as patients with chronic hepatitis C should be treated to prevent progression of cirrhosis and the development of HCC. Combination therapy with peginterferon and ribavirin is the optimal treatment for patients with chronic HCV infection.6 The sustained virologic response (SVR) rate for patients with HCV genotype 1 and HCV genotype 2 or 3, who received peginterferon alpha and ribavirin combination therapy was reported to be 42-46% and 76-80%, respectively.7,8 It was shown that the SVR rate for patients with uncomplicated, biopsy-proven HCV cirrhosis was 33-48%.7,9However, there is little data on patients who have been diagnosed as having HCV cirrhosis by radiological evaluation and/or who have thrombocytopenia or esophageal and gastric varices seen on upper endoscopy.
The aim of this study was to evaluate the SVR rate, the completion rate of treatment, the causes of discontinuation of treatment and the adverse events in patients with clinically diagnosed liver cirrhosis and who received peginterferon and ribavirin combination therapy.
We investigated the patients with HCV-related cirrhosis who Visited 5 tertiary care academic medical centers in Daegu and North Gyeongsang Province from August 2005 to February 2008. The inclusion criteria were (1) positivity for HCV antibodies and RNA, (2) Child-Pugh class A, (3) platelet count >75,000/mm3, (4) AFP level <50 ng/mL and (5) liver cirrhosis diagnosed by radiological evaluation or by detected esophageal and gastric varices on upper gastrointestinal endoscopy. The exclusion criteria were (1) history of decompensated liver cirrhosis (ascites, variceal bleeding, jaundice and hepatic encephalopathy), (2) hepatocelluar carcinoma, (3) HBs Ag positivity and (4) severe depression and severe cardiopulmonary disease not controlled by therapy. Evidence of portal hypertension was defined as presence of esophageal varices and/or by a baseline platelet count <100,000/mm3 and splenomegaly on ultrasonography.
The quantitative HCV-RNA was assessed using Amplicor HCV amplification kit (version 2.0, Roche Molecular diagnostics, IN, USA) and the qualitative HCV-RNA was tested by either Amplicor HCV monitor Kit (version 2.0, Roche Molecular diagnostics, IN, USA) or Abbott real-time kit (Abbott Molecular Inc., Abbott Park, IL, USA). HCV genotyping was performed by VERSANT┬« HCV genotype assay (LiPA, Bayer, NY, USA). The patient with HCV genotype 1 received either peginterferon alpha-2a (40 kd) (Pegasys, Hoffmann-La Roche, Nutley, NJ, USA) 180 ┬Ąg SQ once weekly regardless of their weight or peginterferon alpha-2b (12 kd) (Peg-Intron, Schering- Plough Corporation, Kenilworth, NJ, USA) 1.5 ┬Ąg/kg SQ once weekly plus ribavirin 1,000 mg PO daily for the patients weighting <75 kg or 1,200 mg PO daily for the patients weighting Ōēź75 kg (in 2 divided doses) for 48 weeks. The patients with HCV genotype non-1 received either peginterferon alpha-2a 180 ┬Ąg SQ once weekly regardless of weight or peginterferon alfa-2b 1.5 ┬Ąg/kg SQ once weekly plus ribavirin 800 mg PO daily regardless of weight (in 2 divided doses) for 24 weeks. The dose of peginterferon was reduced if the absolute neutrophil count (ANC) declined to 750/mm3 or the platelet count declined to 50,000/mm3. It was stopped if the ANC declined to 500/mm3 or the platelet count declined to 30,000/mm3. The ribavirin was lowered if the hemoglobin declined to 10.0 g/dL and it was stopped if the hemoglobin declined to 8.0 g/dL.
Early viral response (EVR) was defined as at least a 2 log reduction in the HCV RNA level at 12 weeks of therapy. End of treatment response (ETR) was defined as the absence of detectable HCV-RNA at the end of therapy. Sustained virological response (SVR) was defined as the absence of serum HCV-RNA at the end of therapy and 24 weeks later. Adverse events were assessed by clinical evaluation and laboratory tests at regular intervals.
The data was analyzed by SPSS 13.0 for Window software (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as means┬▒standard deviation (SD) and the differences between the continuous data were analyzed by t-tests. The factors influencing the SVR and the association between the HCV genotype and the ETR or SVR were analyzed by Fisher's exact test and the logistic regression model. A significance level of P=0.05 was used.
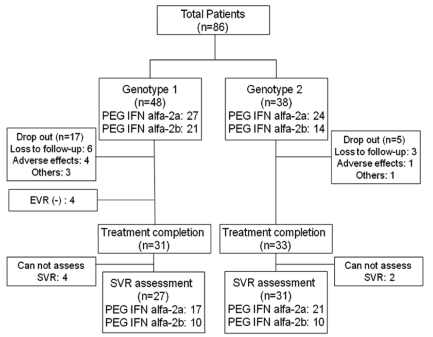
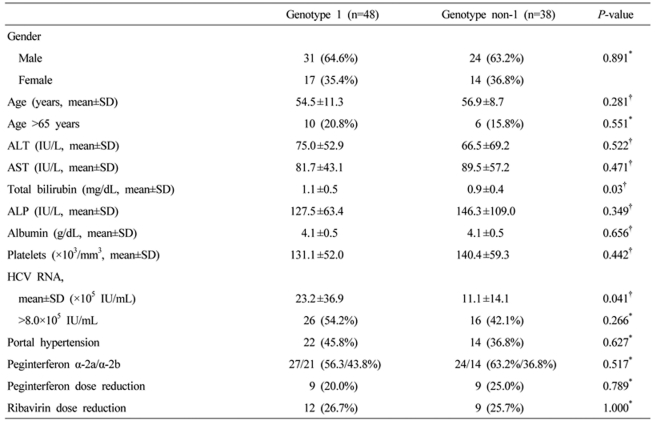
A total of 86 patients were enrolled in this study. Of them, 68 (79.1%) patients completed the treatment (mean age: 56.4┬▒9.6, males: 61.7%, HCV genotype 1: 46.7%) (Fig. 1). A total of 18 patients stopped treatment. The causes of discontinuation of treatment for the HCV genotype 1 patients were adverse events (4 patients, 30.7%), loss to follow-up (6 patients 46.2%), and other problems like lack of money (3 patients, 23.1%). For the HCV genotype non-1 patients, 5 patients stopped the treatment due to loss to follow-up (3 patients, 60%), adverse events (1 patient, 20%) and other problems (1 patient, 20%) (Table 1). There was no patient who stopped the treatment due to hepatic dysfunction or development of complications related to liver cirrhosis. The baseline characteristics in each genotype groups were similar for age, ALT and other characteristics except the baseline serum HCV RNA level and total bilirubin. The baseline serum HCV RNA level and total bilirubin were significantly higher in the genotype 1 patients than those in the genotype non-1 patients (Table 1). Thirty-five patients (51.5%) were diagnosed as liver cirrhosis by radiological evaluation only, and 33 patients (48.5%) were diagnosed by radiologic evaluation, and by presence of esophageal and/or gastric varices on upper gastrointestinal endoscopy. Fifty one patients received peginterferon alpha-2a and 35 patients received peginterferon alpha-2b. The mean duration of follow-up was 23.0┬▒11.9 months from the initial treatment.
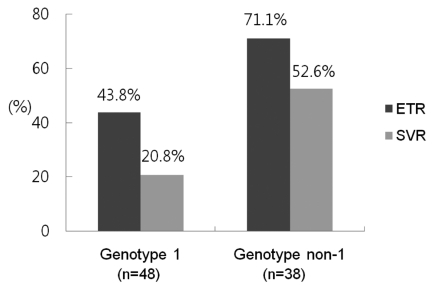
On the intention-to-treat analysis, the overall ETR and SVR rates were 55.8% (48/86) and 34.9% (30/86), respectively. The ETR was achieved in 21/48 (43.8%) and 27/38 (71.1%) of the patients with HCV genotype 1 infection and HCV genotype non-1 infection, respectively (P=0.011). The SVR rates were also lower in HCV genotype 1 infection patients (10/48) than in genotype non-1 infection patients (20/38) (20.8% vs. 52.6%, p=0.002, respectively) (Fig. 2). In per-protocol analysis, Of the 68 patients who completed treatment, the overall SVR rate was 44.1% (30/68). The ETR and SVR rates of the HCV genotype 1 and genotype non-1 patients were 60.0% (21/35) and 28.6% (10/35), and 81.8 (27/33) and 60.6% (20/33), respectively.
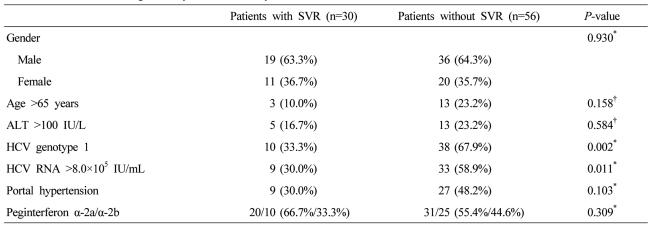
On the univariated analysis, HCV genotype 1 and high baseline viral load (HCV RNA >8.0├Ś105 IU/mL) were associated with low SVR rate (P=0.008, P=0.010) (Table 2). However, old age (>65 years), gender, the baseline alanine aminotransferase (ALT) level, portal hypertension, the types of peginterferon and dose reduction of peginterferon and ribavirin were not associated with the SVR rate. On the multivariated analysis, HCV genotype 1 (P=0.003; odds ratio, 4.464) and high baseline viral load (P=0.012; odds ratio, 3.913) were also independent factors for low SVR rate (Table 3).
Five patients stopped the treatment due to adverse events. The types of adverse events were thrombocytopenia (2 patients), depression (1 patient), anxiety disorder (1 patient) and cerebral vascular hemorrhage (1 patient). The other profiles of adverse events were "flu-like" symptoms, neutropenia, anemia, thrombocytopenia, depression, anorexia, fatigue, myalgia, headache, insomnia, nausea, weight loss, itching, alopecia and rash (Table 4). The peginterferon dose had to be reduced in 18 patients (20.9%) and the ribavirin dose had to be reduced in 21 patients (24.4%). The rate of peginterferon dose reduction was not significantly different between peginterferon alpha 2a (14/51) and alpha 2b (4/35) (27.5% vs. 11.4%, P=0.105). The presence of portal hypertension was not related to dose reduction rates of peginterferon (27.8%, 10/36) and ribavirin (25.0%, 9/36) (P=0.282, P=1.000).
The SVR rate for patients with compensated, biopsy-proven HCV cirrhosis is 34-53%, which was lower than that of the chronic hepatitis patients without cirrhosis.7-8,12 The SVR rate for HCV cirrhotic patients with portal hypertension is 21.6%.13 In our study, the overall SVR rate of patients who were clinically diagnosed as cirrhosis was 34.9%. This was lower than that of the patients who were diagnosed as cirrhosis through liver biopsy in other studies,14 but slightly higher than those of studies on patients with portal hypertension. Liver histological examination based on percutaneous needle biopsy is considered as a gold standard method for diagnosis of liver cirrhosis. However, liver biopsy cannot be performed routinely at clinical practice because of complication risk and patients' discomforts. Therefore, in actual clinical setting, radiologic examinations such as abdominal ultrasonography or computed tomography are regularly performed for viral hepatitis patients. These radiologic examinations are difficult to clearly distinguish between chronic hepatitis and cirrhosis. However, there are many reports saying that specificity is very high although sensitivity is relatively low, when there are such manifestations as irregular liver surface, nodular liver parenchyma, atrophy of right hepatic lobe, hypertrophy of caudate lobe and left hepatic lobe, and splenomegaly.10,11 Therefore, if the abdomen US or CT finds the typical manifestations of cirrhosis, it means that the patient is highly likely to have liver cirrhosis. In clinical settings, most patients who are diagnosed as liver cirrhosis by radiologic imaging or endoscopic finding are likely to be treated with peginterferon and ribavirin without liver biopsy.
In cirrhotic patients, the SVR rate of the genotype non-1 group was similar at around 52.6%. The SVRs of genotype 1 group was reported to be very low at 13-14%,14,15 however, the SVR was relatively high at 20.8% in this study. This was consistent with the results of relatively high SVRs in Korean patients compared to those in Western countries in studies with patients who did not have liver cirrhosis, but the accurate reason is unknown.16,17 Furthermore, in this study, even the SVR rate of patients with portal hypertension who showed splenic enlargement in imaging study, thrombocytopenia, or esophageal or gastric varices in upper gastrointestinal endoscopy was 30.0% (9/30), which was better than the results of other studies. There was no significant difference in SVR, compared to patients who did not have portal hypertension. However, the clinical judgment on portal hypertension may be subjective and the number of patients is small in our study. Therefore, accurate diagnosis methods such as measurement of hepatic venous pressure gradient for the assessment of portal hypertension, and studies with a larger number of patients may be needed.
It is generally well-known that chronic hepatitis C patients without cirrhosis show low SVR if they have HCV genotype 1 and have high viral load before the treatment. It has also been reported that these factors are associated with SVR in patients with compensated liver cirrhosis.8,18,19 The present study also found that only these two factors were associated with SVR, which is consistent with the results of other studies.
In our study, there were 5 patients (5.8%, 5/86) who discontinued treatment due to adverse reactions of peginterferon and ribavirin combination therapy. This was similar to about 6.0-10.3% reported in domestic studies on chronic hepatitis C patients,16,17 but lower than 16.0-32.3% reported in studies on patients who were accompanied by compensated liver cirrhosis or portal hypertension.13,14 However, simple comparison is not warranted because this was a retrospective study, and there is the possibility that some of the 9 patients (10.5%) who were not followed up without particular reason may voluntarily discontinued the treatment due to other side effects than hematological adverse reactions. Nevertheless, as treatment stopped in less than 20% of patients when these patients were included, the number of cases who stopped treatment due to adverse reactions of peginterferon and ribavirin combination therapy in patients with cirrhosis was similar to that of chronic hepatitis C patients. Of 86 patients, the patients who reduced peginterferon and ribavirin due to hematological adverse reactions were 20.9% (18/86) and 24.4% (21/86), respectively. These rates of dose reduction were not significantly greater in patients with portal hypertension, although hematologic adverse reaction expected to be more frequent.
As the limitation of retrospective study, we could not follow up every patients who had started the treatment to the completion of treatment, and the medical records were not standardized and consistent by organization. Therefore, so it is difficult to arrive at final conclusions. However, even schematically, this study confirmed the expected results and possible adverse reactions of the peginterferon and ribavirin combination therapy in chronic hepatitis C patients who were clinically diagnosed with cirrhosis.
In conclusion, the overall SVR can be achieved in one-third of clinically diagnosed cirrhosis patients with HCV infection. Especially for patients with genotype non-1 infection, the SVR rate was high. Therefore, planning treatment on the basis of viral genotype and baseline viral load are needed to select patients who are more likely to achieve SVR.
REFERENCES
1. Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat 1999;6:35-47. 10847128.


4. Poynard T, McHutchison J, Davis GL, Esteban-Mur R, Goodman Z, Bedossa P, et al. Impact of interferon alfa-2b and ribavirin on progression of liver fibrosis in patients with chronic hepatitis C. Hepatology 2000;32:1131-1137. 11050066.


5. Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, et al. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med 2005;142:105-114. 15657158.


6. Strader DB, Wright T, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology 2004;39:1147-1171. 15057920.


7. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gon├¦ales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-982. 12324553.


8. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958-965. 11583749.


9. Braks RE, Ganne-Carrie N, Fontaine H, Paries J, Grando-Lemaire V, Beaugrand M, et al. Effect of sustained virological response on long-term clinical outcome in 113 patients with compensated hepatitis C-related cirrhosis treated by interferon alpha and ribavirin. World J Gastroenterol 2007;13:5648-5653. 17948941.



10. Goyal N, Jain N, Rachapalli V, Cochlin DL, Robinson M. Non-invasive evaluation of liver cirrhosis using ultrasound. Clin Radiol 2009;64:1056-1066. 19822238.


11. Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection-analysis of 300 cases. Radiology 2003;227:89-94. 12601199.


12. Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, et al. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology 2010;51:388-397. 19918980.


13. Di Marco V, Almasio PL, Ferraro D, Calvaruso V, Alaimo G, Peralta S, et al. Peg-interferon alone or combined with ribavirin in HCV cirrhosis with portal hypertension: a randomized controlled trial. J Hepatol 2007;47:484-491. 17692985.


14. Giannini EG, Basso M, Savarino V, Picciotto A. Predictive value of on-treatment response during full-dose antiviral therapy of patients with hepatitis C virus cirrhosis and portal hypertension. J Intern Med 2009;266:537-546. 19849774.


15. Syed E, Rahbin N, Weiland O, Carlsson T, Oksanen A, Birk M, et al. Pegylated interferon and ribavirin combination therapy for chronic hepatitis C virus infection in patients with Child-Pugh Class A liver cirrhosis. Scand J Gastroenterol 2008;43:1378-1386. 18615358.


16. Kim KT, Han SY, Kim JH, Yoon HA, Baek YH, Kim MJ, et al. Clinical outcome of pegylated interferon and ribavirin therapy for chronic hepatitis C. Korean J Hepatol 2008;14:36-45. 18367856.


17. Kim JI, Kim SH, Lee BS, Lee HY, Lee TH, Kang YW, et al. Efficacy of initial treatment with peginterferon alpha-2a versus peginterferon alpha-2b in combination with ribavirin in naive chronic hepatitis C patients living in Daejeon and Chungcheong Province in Korea: a comparative study. Korean J Hepatol 2008;14:493-502. 19119244.


18. Furusyo N, Hayashi J, Kashiwagi K, Nakashima H, Nabeshima S, Sawayama Y, et al. Hepatitis C virus (HCV) RNA level determined by second-generation branched-DNA probe assay as predictor of response to interferon treatment in patients with chronic HCV viremia. Dig Dis Sci 2002;47:535-542. 11911338.


Figure┬Ā2
End of treatment response (ETR) and SVR rates according to HCV genotypes. ETR and SVR rates were significantly higher in patients with genotype non-1 than in those with genotype 1 (71.1% vs. 43.8%, P=0.011; and 52.6% vs. 20.8%, P=0.002, respectively).





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




