| Clin Mol Hepatol > Volume 30(2); 2024 > Article |
|
See the letter "Reply to: ŌĆ£Evaluation of the histological scoring systems of autoimmune hepatitis: A significant step towards the optimization of clinical diagnosisŌĆØ" on page 291.
See the Original "Comparison of four histological scoring systems for autoimmune hepatitis to improve diagnostic sensitivity" on page 37.
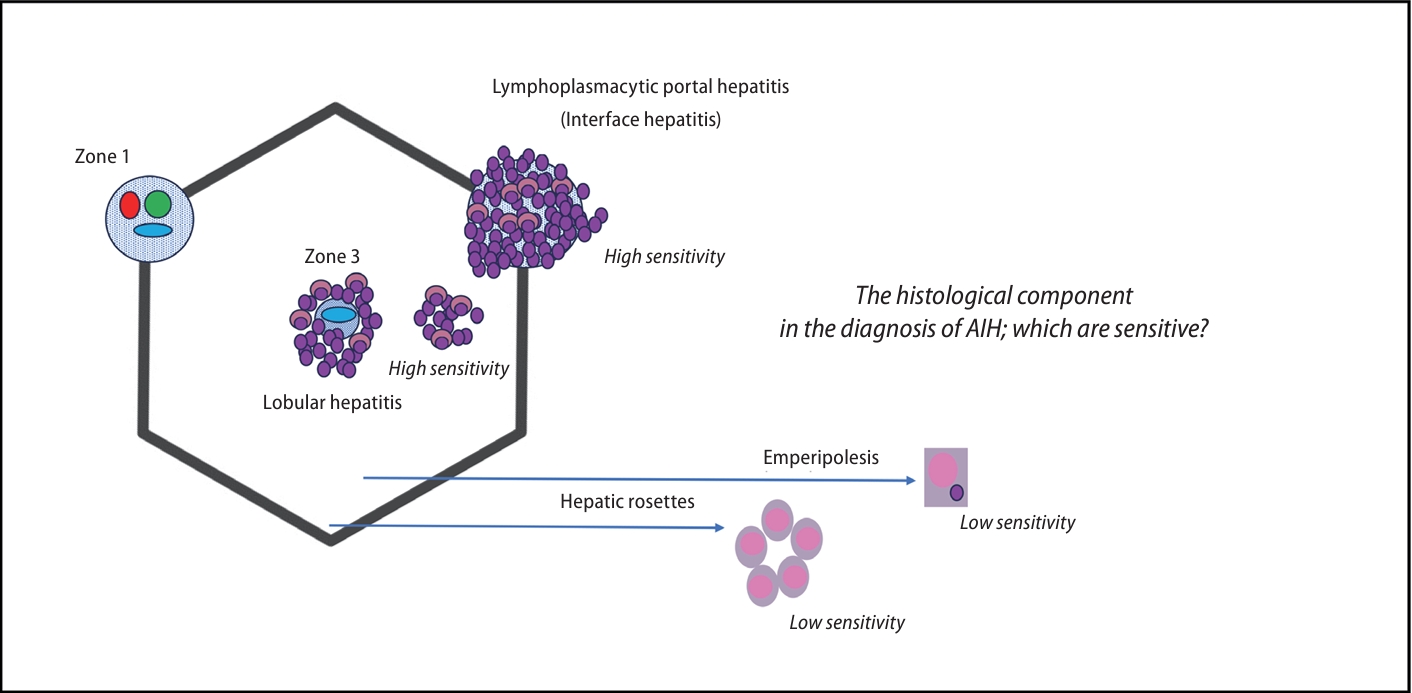
Autoimmune hepatitis (AIH) is an immune-inflammatory chronic liver disease in nature; however, clinicians are well aware of heterogenous disease phenotypes, not to mention chronological dynamics: from acute-onset and acute-onchronic to chronic insidious manifestation [1]. For many years, there were two commonly implemented sets of AIH diagnostic criteria: the revised International AIH study group criteria reported in 1999 (hereafter referred to as ŌĆ£1999 IAIHGŌĆØ) [2] and the simplified criteria proposed in 2008 (or ŌĆ£2008 IAIHGŌĆØ) [3]. The 2008 IAIHG system has superior specificity and accuracy compared to the 1999 IAIHG system, while the 1999 IAIHG system is regarded as suitable for the assessment of atypical cases, including acute-onset AIH [4]. Their differences may stem from the fact that the histological component of the 2008 IAIHG system emphasizes emperipolesis and hepatic rosette formation as typical features supporting the diagnosis of AIH. Recently, however, emperipolesis and rosettes have instead come to be considered etiology-independent reflections of lymphocyte-driven unicellular injury on the one hand and multicellular regeneration of hepatocytes on the other [5]. In other words, pathologists have questioned the diagnostic specificity of these two features and more recently proposed modifications to the histological component of the 2008 IAIHG criteria, discarding those two features and adding lobular hepatitis instead. The outcome of these efforts was the 2017 UCSF criteria by Balitzer et al. [6], followed by the consensus statement for the histological diagnosis of AIH by the International Autoimmune Hepatitis Pathology Group (2022 IAHPG) [7].
In this issue, Ahn et al. [8] retrospectively evaluate the utility of the two modified histological scorings among patients originally diagnosed with at least probable AIH according to 1999 IAIHG by performing a comparison among four sets of diagnostic criteria, namely 1999 IAIHG, 2008 IAIHG, and 2008 IAIHGs, with the updated histological scorings of 2017 UCSF and 2022 IAHPG, respectively (2008 IAIHG+2017 UCSF and 2008 IAIHG+2022 IAHPG).
First, as expected, the percentage among the cohort who received the maximum histology score was lower for 2008 IAIHG than for 1999 IAIHG (58.8% vs. 73.5%), while implementation of either 2017 UCSF or 2022 IAHPG criteria with 2008 IAIHG increased the percentage to 88.2% or 94.1%, respectively. Accordingly, the percentage of patients who met probable and definite (Ōēźprobable) AIH was 89.7% and 91.2% by 2008 IAIHG+2017 UCSF and 2008 IAIHG+2022 IAHPG, respectively, compared with 82.4% by 2008 IAIHG.
Second, and relevantly for clinicians, their sub-analysis focused on the re-evaluation of cases with acute onset or aggravation (AIH with acute presentation). AIH with acute presentation is a challenging disease phenotype because a delayed or insensitive diagnosis may lead to a worse prognosis, especially in cases with atypical serological findings [1]. Moreover, the histological features of AIH with acute presentation do not always exhibit, nor are they limited to, the classical chronic hepatitis features of lymphoplasmacytic portal infiltrates with interface hepatitis: core histological components in both IAIHG systems [2,3]. As mentioned above, 2017 UCSF and 2022 IAHPG, in particular, adopted lobular hepatitis as a histological component in order to strengthen the diagnostic power, even for AIH with acute presentation [6,7]. Again, as anticipated, the percentage receiving the maximum histology score among AIH with acute presentation increased from 65.6% in 2008 IAIHG to 90.6% in 2008 IAIHG+2017 UCSF, and further to 96.9% in 2008 IAIHG+2022 IAHPG. Consequently, the percentage of patients who met probable or definite (i.e., Ōēźprobable) AIH criteria was 81.3, 90.6, and 93.8% by 2008 IAIHG, 2008 IAIHG+2017 UCSF, and 2008 IAIHG+2022 IAHPG, respectively.
The fact that AIH criteria considering lobular hepatitis (in addition to lymphoplasmacytic interface hepatitis) outcompete those considering emperipolesis and hepatic rosette formation reinforces the position that the context of demarcated hepatic inflammation or liver zonation likely matters in the multifaceted pathology of AIH (Fig. 1). Practical technical issues around pathological diagnosis may also support their findings. Recognition of emperipolesis, the presence of lymphocytes within the cytoplasm of hepatocytes, is often a difficult, time-consuming task, in particular by general pathologists, with a tendency for wide interobserver variability [5]; the frequency of emperipolesis varies and has been reported as low as ~15% in even a recent national survey in Japan [9].
AIH is a disease without signature diagnostic features. A specific pathological signature is still being sought, the latest candidate being Kupffer cell hyaline globules [10]. Kupffer cells in the inflamed liver have been speculated to catabolize and recycle excess immunoglobulins (Ig) secreted by plasma cells, giving rise to hyaline globules within the cytoplasm. Kupffer cell hyaline globules have been reported to be relatively specific in AIH compared with other liver diseases [5], but it is still controversial for their disease specificity and association with serum Ig levels [11]. The need to do additional staining to identify Kupffer cells is a drawback of its routine implementation in clinical practice. So far, again, understanding the specific context of AIH inflammation in situ is the gold standard in AIH diagnosis, as in the findings by Ahn et al. [8]; there is likely room for improvement in the future with assistance from artificial intelligence.
The study by Ahn et al. [8] was a retrospective study with only a relatively small number of Korean AIH patients in the absence of a control group with other etiologies. In the future, multinational evaluation to validate the sensitivity and assess the specificity of the four diagnostic systems will be important, especially one that considers acute drug-induced liver injury, a most prominent disease entity that requires careful differentiation from AIH with acute presentation [12].
REFERENCES
1. Komori A. Recent updates on the management of autoimmune hepatitis. Clin Mol Hepatol 2021;27:58-69.




2. Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929-938.


3. Hennes EM, Zeniya M, Czaja AJ, Par├®s A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169-176.


4. Czaja AJ. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatology 2008;48:1540-1548.


5. Zhang X, Jain D. The many faces and pathologic diagnostic challenges of autoimmune hepatitis. Hum Pathol 2023;132:114-125.


6. Balitzer D, Shafizadeh N, Peters MG, Ferrell LD, Alshak N, Kakar S. Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Mod Pathol 2017;30:773-783.



7. Lohse AW, Sebode M, Bhathal PS, Clouston AD, Dienes HP, Jain D, et al. Consensus recommendations for histological criteria of autoimmune hepatitis from the International AIH Pathology Group: Results of a workshop on AIH histology hosted by the European Reference Network on Hepatological Diseases and the European Society of Pathology: Results of a workshop on AIH histology hosted by the European Reference Network on Hepatological Diseases and the European Society of Pathology. Liver Int 2022;42:1058-1069.

8. Ahn S, Jeong SH, Cho EJ, Lee K, Kim G, Kim H. Comparison of four histological scoring systems for autoimmune hepatitis to improve diagnostic sensitivity. Clin Mol Hepatol 2024;30:37-48.




9. Takahashi A, Arinaga-Hino T, Ohira H, Torimura T, Zeniya M, Abe M, et al. Autoimmune hepatitis in Japan: trends in a nationwide survey. J Gastroenterol 2017;52:631-640.



10. Tucker SM, Jonas MM, Perez-Atayde AR. Hyaline droplets in Kupffer cells: a novel diagnostic clue for autoimmune hepatitis. Am J Surg Pathol 2015;39:772-778.

11. Himoto T, Kadota K, Fujita K, Nomura T, Morishita A, Yoneyama H, et al. The pathological appearance of hyaline droplets in Kupffer cells is not specific to patients with autoimmune hepatitis. Int J Clin Exp Pathol 2017;10:8703-8708.


12. Tsutsui A, Harada K, Tsuneyama K, Nguyen Canh H, Ando M, Nakamura S, et al. Histopathological analysis of autoimmune hepatitis with ŌĆ£acuteŌĆØ presentation: Differentiation from drug-induced liver injury. Hepatol Res 2020;50:1047-1061.



-
METRICS

- ORCID iDs
-
Atsumasa Komori

https://orcid.org/0000-0002-2607-9381 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



