| Clin Mol Hepatol > Volume 29(Suppl); 2023 > Article |
|
ABSTRACT
FOOTNOTES
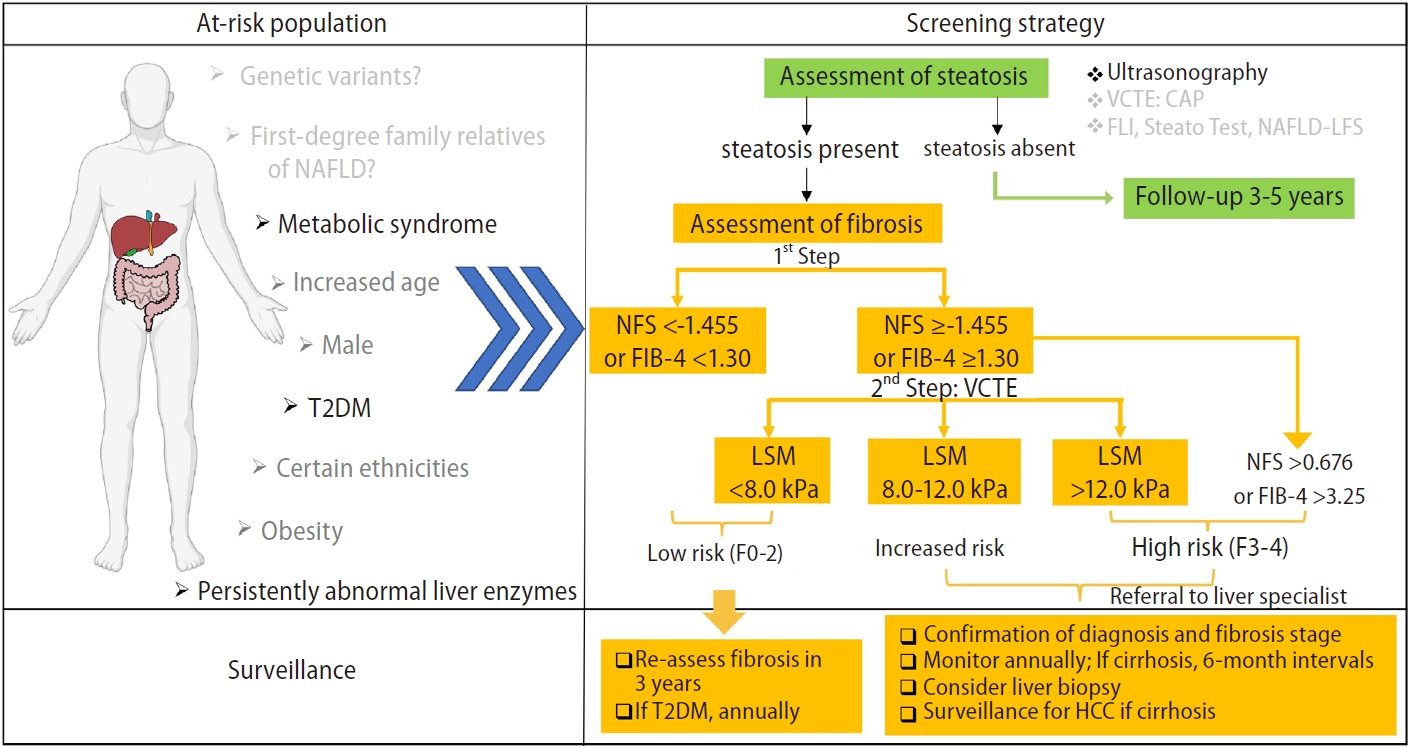
Figure┬Ā1.
Table┬Ā1.
| Professional organizations | Year | Guidance statements |
|---|---|---|
| European Association for the Study of Liver (EASL), European Association for the Study of Diabetes (EASD), and European Association for the Study of Obesity (EASO) [12] | 2016 | Screening for NAFLD in people with obesity, metabolic syndrome, and in particular, T2DM |
| American Association for the Study of Liver Diseases (AASLD) [13] | 2018 | 1. Routine screening for NAFLD in high-risk populations (obesity, T2DM) is not advised due to uncertainties in diagnostic testing, long-term management, and cost-effectiveness |
| 2. Endorses ŌĆ£vigilanceŌĆØ in patients with T2DM | ||
| 3. Systematic screening of family members for NAFLD is not currently recommended | ||
| Asian Pacific Association for the Study of the Liver (APASL) [14] | 2020 | Screening in those with T2DM or metabolic syndrome, or those who are overweight/obese according to ethnic-specific cut-offs |
| The American Academy of Pediatrics [17-19] | 2007; 2014; 2017 | 1. Currently, the best screening test for NAFLD in children is ALT; however, it has substantial limitations. |
| 2. Screening should be considered for obese youth with additional risk factors (central adiposity, insulin resistance, pre-diabetes or diabetes, dyslipidemia, sleep apnea, or family history of NAFLD/ NASH) | ||
| 3. Follow-up screening for NAFLD is recommended. When the initial screening test is normal, consider repeating ALT every 2ŌĆō3 years if risk factors remain unchanged | ||
| The American Diabetes Association (ADA) [20] | 2019 | Patients with type 2 diabetes or prediabetes and elevated liver enzymes (ALT) or fatty liver on ultrasound should be evaluated for the presence of non-alcoholic steatohepatitis and liver fibrosis |
| Korean Association for the Study of the Liver (KASL) [15] | 2021 | 1. Subjects who have persistent liver enzyme elevation, metabolic syndrome, or diabetes should be screened for NAFLD |
| 2. Abdominal ultrasonography is the primary screening modality | ||
| British Association for the Study of the Liver (BASL) and British Society of Gastroenterology (BSG) NAFLD Special Interest Group [16] | 2022 | 1. Services should have an agreed local clinical pathway for the investigation of suspected liver disease |
| 2. Consider the possibility of liver fibrosis due to NAFLD in people with T2DM or metabolic syndrome |
Table┬Ā2.
| Populations | Supporting screening | Guidelines | Against screening | Guidelines |
|---|---|---|---|---|
| Age >50 years | ŌłÜ | 2016 EASL-EASD-EASO | ||
| Obesity | ŌłÜŌłÜ | 2016 EASL-EASD-EASO | X | 2018 AASLD |
| 2019 APASL | ||||
| Type 2 diabetes | ŌłÜŌłÜŌłÜ | 2016 EASL-EASD-EASO | X | 2018 AASLD |
| 2019 APASL | ||||
| 2021 KASL | ||||
| Metabolic syndrome | ŌłÜŌłÜŌłÜ | 2016 EASL-EASD-EASO | ||
| 2019 APASL | ||||
| 2021 KASL | ||||
| Persistently abnormal liver enzymes | ŌłÜŌłÜŌłÜ | 2016 EASL-EASD-EASO | ||
| 2019 APASL | ||||
| 2021 KASL | ||||
| Obese youth with additional risk factors* | ŌłÜ | The American Academy of ediatrics | ||
| First-degree relatives of NAFLD | XX | 2016 EASL-EASD-EASO | ||
| 2018 AASLD | ||||
| Genetic variants | XX | 2016 EASL-EASD-EASO | ||
| 2018 AASLD |
NAFLD, non-alcoholic fatty liver disease; EASL, European Association for the Study of Liver; EASD, European Association for the Study of Diabetes; EASO, European Association for the Study of Obesity; APASL, Asian Pacific Association for the Study of the Liver; KASL, Korean Association for the Study of the Liver; AASLD, American Association for the Study of Liver Diseases; NASH, nonalcoholic steatohepatitis.
ŌłÜ indicated the number of guidelines that support screening for NAFLD in this population. X indicated the number of guidelines against screening for NAFLD in this population. The number of markers indicate the strength of recommendation.
Table┬Ā3.
| Diagnostic panel | Cost | Features |
Detection abilities |
|||
|---|---|---|---|---|---|---|
| Steatosis | Advanced fibrosis | Cirrhosis | ||||
| Serological markers | ||||||
| Fatty liver index [85] | $ | Common parameters involved (BMI, WC, triglycerides, and GGT) | ŌłÜ | X | X | |
| Cannot distinguish between steatosis grades | ||||||
| Hepatic steatosis index [86] | $ | Common parameters involved (AST: ALT ratio, BMI, female sex, and DM) | ŌłÜ | X | X | |
| Inadequate distinction of the severity of steatosis | ||||||
| SteatoTest [87,88] | $\$ | Involves biomarkers that are not routinely done (╬▒2M, haptoglobin, ApoA-1, total bilirubin, GGT, fasting glucose, triglycerides, cholesterol, and ALT, adjusted for patient's age, sex, weight, and height) | ŌłÜ | X | X | |
| FIB-4 [94] | $ | A formula comprising age, platelet, AST, and ALT | X | ŌłÜ | ŌłÜ | |
| One of the best non-invasive tests for diagnosing advanced fibrosis in NAFLD | ||||||
| Rules out advanced fibrosis | ||||||
| NFS [95-97] | $ | A formula comprising age, hyperglycemia, BMI, platelet count, albumin, and AST/ALT ratio | X | ŌłÜ | ŌłÜ | |
| Identifies advanced fibrosis well | ||||||
| Needs independent adjustment of BMI across ethnic groups | ||||||
| BARD score [95] | $ | A formula comprising BMI, AST/ALT ratio, and diabetes | X | ŌłÜ | ŌłÜ | |
| Does not predict fibrosis well in patients with mild NAFLD (specifically in patients with obesity or T2DM), which limits its clinical use | ||||||
| ELF [89-91] | $\$ | Consists of an algorithm of three fibrosis markers (HA, PIIINP, and TIMP-1) that are not routinely measured | X | ŌłÜ | ŌłÜ | |
| Rules out advanced fibrosis | ||||||
| FibroTest [87,92,93] | $\$ | Involves biomarkers that are not routinely done (╬▒2M, haptoglobin, ApoA-1, total bilirubin, GGT) | X | ŌłÜ | X | |
| Affected by other causes of hyperbilirubinemia and elevated GGT | ||||||
| Imaging modalities | ||||||
| Ultrasonography [99-101] | $ | AUROC 0.97 good predictive tool for steatosis but does not provide information regarding fibrosis, unless cirrhosis is established | ŌłÜ | X | ŌłÜ | |
| VCTE [105-107,111] | $ | AUROC 0.84 for F2 fibrosis with the M probe | ŌłÜ ŌłÜ | ŌłÜ ŌłÜ | ŌłÜ ŌłÜ | |
| AUROC 0.93 for F3 fibrosis with the M probe | ||||||
| AUROC 0.95 for F4 fibrosis with the M probe | ||||||
| AUROC 0.80ŌĆō0.85 for F2 fibrosis with the XL probe | ||||||
| AUROC 0.84ŌĆō0.90 for F3 fibrosis with the XL probe | ||||||
| AUROC 0.91ŌĆō0.95 for F4 fibrosis with the XL probe | ||||||
| Not accurate in patients with cholestasis, ascites, and congestive heart failure | ||||||
| MRI-PDFF [110-112] | $\$$ | Good specificity and sensitivity in detecting steatosis | ŌłÜ ŌłÜ | X | X | |
| Less reliable for grading steatosis in patients with advanced fibrosis or cirrhosis | ||||||
| Cannot be performed in patients with claustrophobia, and the measurements are affected by hepatic iron deposition | ||||||
| Not widely available | ||||||
| MRS [112] | $\$$ | Results of this tool might be affected by respiration movements, claustrophobia, and implanted devices | ŌłÜ ŌłÜ ŌłÜ | X | X | |
| Only available in specialized centers | ||||||
| MRE [110,113-116] | $\$$ | AUROC 0.86ŌĆō0.89 for F2 fibrosis | X | ŌłÜ ŌłÜ ŌłÜ | ŌłÜ ŌłÜ ŌłÜ | |
| AUROC 0.89ŌĆō0.96 for F3 fibrosis | ||||||
| AUROC 0.88ŌĆō0.97 for F4 fibrosis | ||||||
| Accessibility is limited by requirement of specific scanner hardware | ||||||
| SWE [88,117,118] | $ | No well-established cutoffs for NAFLD | X | ŌłÜ ŌłÜ | ŌłÜ ŌłÜ | |
| Results may differ from liver biopsy; accurate if >30% of hepatocytes are steatotic | ||||||
| Reduced sampling errors | ||||||
NAFLD, non-alcoholic fatty liver disease; BMI, body mass index; WC, waist circumference; GGT, gammaŌĆÉglutamyltransferase; DM, diabetes mellitus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FIB-4, fibrosis-4; NFS, NAFLD fibrosis score; T2DM, type 2 diabetes mellitus; ELF, enhanced liver fibrosis; AUROC, area under the receiver operating characteristic curve; VCTE, vibration-controlled transient elastography; MRI-PDFF, magnetic resonance imaging-estimated proton density fat fraction; MRS, magnetic resonance spectroscopy; MRE, magnetic resonance elastography; SWE, shear wave elastography; ╬▒2M, ╬▒2-macroglobulin; ApoA-1, Apolipoprotein AI; BARD, body mass index, AST/ALT ratio, and diabetes; HA, hyaluronic acid; PIIINP, type III procollagen peptide; TIMP-1, tissue inhibitor of metalloproteinases-1.
$ indicated the relative cost of using this method for NAFLD screening. $, relatively low; $\$, relatively medium; $\$$, relatively high. ŌłÜ indicated the relative detection abilities of this method. $, relatively low; ŌłÜ, relatively medium; ŌłÜ, relatively high. X indicated that this screening method could not detect steatosis, advanced fibrosis, or cirrhosis.
Table┬Ā4.
| Developing modalities | Components | AUROC | Comments | |
|---|---|---|---|---|
| Serum-based | Perilipin-2 (PLIN2) [119] mean fluorescence intensity | Combined with waist circumference, triglyceride, ALT and presence/absence of diabetes as covariates as a biomarker for NASH | An accuracy of 93% in the discovery cohort and 92% in the validation cohort | Using flow cytometry to measure PLIN2 in peripheral blood monocytes |
| Current form not feasible for screening | ||||
| Ras-related protein (RAB14) [119] mean fluorescence intensity | Combined with age, waist circumference, high-density lipoprotein cholesterol, plasma glucose, and ALT levels as covariates as a biomarker for NASH | 99.3%, significantly higher than NFS (85.2%), FIB-4 (62.2%), APRI (61.8%) | Using flow cytometry to measure RAB14 in peripheral blood monocytes | |
| Current form not feasible for screening | ||||
| Thrombospondin-2 (TSP2) [120] | A novel fibrosis biomarker of NAFLD in T2DM | 0.80, indicating fibrosis ŌēźF3 on VCTE, superior to both FIB-4 and NFS | Existing commercial enzyme- linked Immuno-sorbent Assay | |
| Cutoff: 3.6 ng/mL to identify ŌēźF3 fibrosis | ||||
| Lipocalin-2 (LCN2) [121] | A valuable NAFLD biomarker, especially for the transition from NAFL to NASH | AUC: 0.987 for NASH diagnosis, and AUC: 0.977 for steatosis | Unable to establish an optimal cut-off value for distinguishing NASH from NAFL | |
| Using a rapid, portable, point-of- care, and user-friendly point-of- care assay | ||||
| Metabolomics | Amino acids [123,124] | The ratio of glutamate/(serine+glycine) | F0ŌĆōF2 vs. F3ŌĆōF4, highest odds ratio (OR) for liver fibrosis (F3ŌĆō4) | Using gas chromatography-mass spectrometry |
| Current form not feasible for screening | ||||
| Bile acids [124,125] | 7-ketodeoxycholic acid (7-Keto-DCA) | Advanced fibrosis (OR, 4.2), NASH (OR, 24.5), and hepatocellular ballooning (OR, 18.7) | Biomarkers for NAFLD progression | |
| Independent validation is required | ||||
| Using a stable isotope-dilution LC- MS/MS method | ||||
| Current form not feasible for screening | ||||
| 7-ketolithocholic acid (7-Keto-LCA) | NASH (OR, 9.4) and ballooning (OR, 5.9) | |||
| Stool-based | Fecal-microbiome derived metagenomic signature [126] | 37 bacterial species are used to construct a Random Forest classifier model to detect advanced fibrosis in NAFLD | A robust diagnostic accuracy (AUC 0.936) | Need to utilize metagenomics sequencing |
| Current form not feasible for screening | ||||
| Device-based | Multi-spectral EIT [122] | Using waist-over-height biometric as complementary information | Predict clinicalstandard CAP in patients with or without NAFLD | Portable |
| Self-administrable | ||||
| Potentially cost-effective and with a short acquisition time (3 minutes) | ||||
| Only with pilot results, need validation in large cohorts | ||||
| 13C-methacetin breath test [127,128] | Quantitative evaluation of the cytochrome P450-dependent liver function | A good tool for identifying patients with histologically proven NASH (AUROC: 0.824); | Separate patients with normal/NAFL from patients with NASH | |
| Fail to detect early stages of fibrosis | ||||
| Predicts F3 or F4 fibrosis (AUROC: 0.936 and 0.973) | Mainly investigated in patients with chronic hepatitis C | |||
NAFLD, non-alcoholic fatty liver disease; AUROC, area under the receiver operating characteristic curve; ALT, alanine aminotransferase; NASH, nonalcoholic steatohepatitis; NFS, NAFLD fibrosis score; FIB-4, fibrosis-4; AST, aspartate aminotransferase; APRI, AST to platelet ratio index; T2DM, type 2 diabetes mellitus; VCTE, vibration-controlled transient elastography; AUROC, the area under a receiver operating characteristic curve; AUC, area under the curve; EIT, electrical impedance tomography; CAP, controlled attenuation parameter; LC-MS/MS, liquid chromatography-mass spectrometry/mass spectrometry.
Abbreviations
REFERENCES
-
METRICS

- ORCID iDs
-
Wai-Kay Seto

https://orcid.org/0000-0002-9012-313X - Related articles
-
Recent updates on pharmacologic therapy in non-alcoholic fatty liver disease2024 January;30(1)
Letter regarding ŌĆ£Risk factors in nonalcoholic fatty liver diseaseŌĆØ2023 October;29(4)
Lean or Non-obese Nonalcoholic Fatty Liver Disease Patients: Are They Really Lean?2023 October;29(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



