| Clin Mol Hepatol > Volume 28(3); 2022 > Article |
|
See the commentary-article "A crystal ball to forecast treatment responsiveness in nonalcoholic fatty liver disease" on page 478.
ABSTRACT
Background/Aims
We aimed to define an optimal target population and drug-specific biomarkers that may predict dipeptidyl peptidase (DPP)-4 inhibitor responses in non-alcoholic fatty liver disease (NAFLD).
Methods
An exploration study (study I) was performed using three different NAFLD models (basket study design; high-fat diet [HFD], methionine choline-deficient diet [MCD], and high-cholesterol Western diet [WD] models). RNA transcriptome analysis was performed on pre-studied liver tissues to identify biomarkers that could predict the response to DPP-4 inhibitors. In the validation study (study II), the HFD-induced NAFLD model was divided into high and low hepatic insulin-like growth factor binding protein 1 (Igfbp-1) groups based on the pre-study liver biopsy.
Results
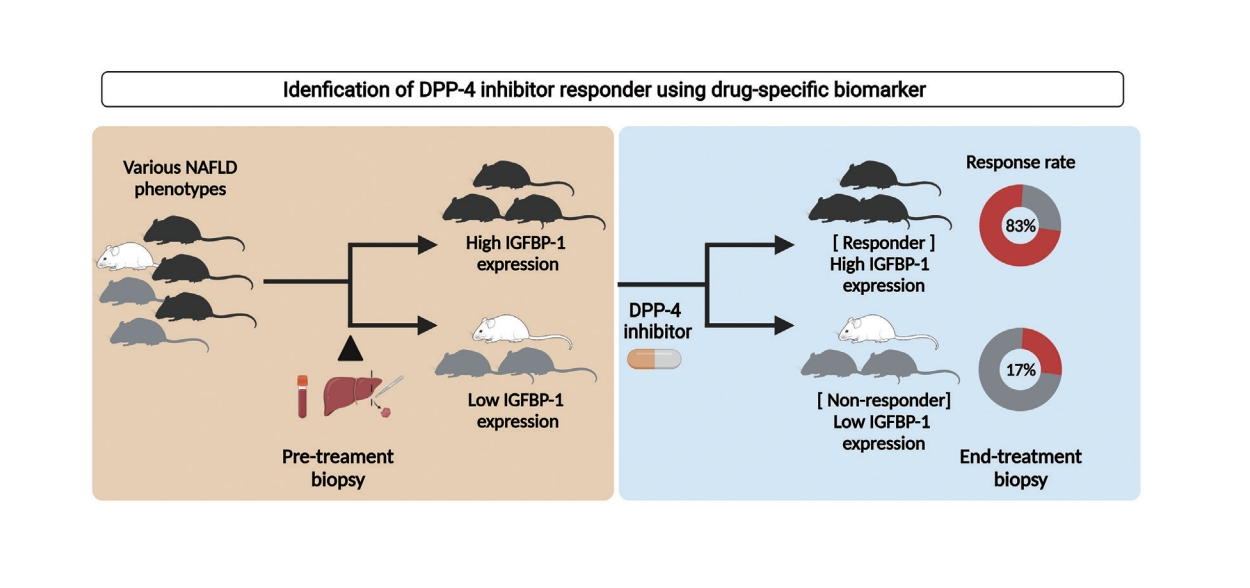
DPP-4 inhibitor attenuated the NAFLD activity score and fibrosis stage in the HFD model but not in the WD and MCD models. The overall response rate was 19% across the modified basket NAFLD trial and 42%, 25%, and 0% in the HFD, WD, and MCD models. Hepatic Igfbp-1 expression was higher in the responder group than in the non-responder group in pre-study biopsy samples. In contrast, hepatic Igfbp-1 expression was lower in the responder group than in the non-responder group in the end-study biopsy samples. DPP-4 inhibitor response rates were 83% and 17% in the baseline hepatic high Igfbp-1 and low Igfbp-1 groups, respectively. Hepatic messenger RNA Igfbp-1 expression was positively correlated with serum IGFBP-1 levels.
Graphical Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease. The overall prevalence of NAFLD in Asian countries is approximately 29.6% [1]. Approximately 30% of NAFLD cases are accompanied by steatohepatitis or fibrosis, leading to the progression of liver disease. Annual medical costs and the socioeconomic burden associated with NAFLD have increased sharply in the United States [2].
No drugs have been approved for the treatment of NAFLD. Several phase IIb and III clinical trials are ongoing; however, the results are unsatisfactory [3]. One of the main reasons for this is the heterogeneity of the patients with NAFLD. There are diverse phenotypes and complex pathophysiologies in patients with NAFLD. There are diverse phenotypes and complex pathophysiologies in patients with NAFLD, which must be targeted precisely to treat them effectively [4,5]. However, current clinical trials are not based on precision medicine because of the lack of biomarkers to assess the response. Therefore, the response rate of most current clinical trials is less than 40% [4,6,7]. Identifying drug-specific biomarkers is a necessity and a first step toward overcoming this challenge. It has become imperative to discover biomarkers that can identify the optimal target population and predict the therapeutic response of the drug at a preclinical stage.
Recently, two new strategies have been proposed for clinical trials in personalized medicine. The first trial design, called the basket trial, is to determine drugs that are effective against various types of cancers, and the second, called the umbrella trial, is to determine the efficacy of the various drugs for a specific type of disease condition [8]. Therefore, this study was conducted in two stages to find a drug-specific biomarker for a dipeptidyl peptidase (DPP)-4 inhibitor. First, we attempted to find the optimal target population by using the best of three kinds of animal NAFLD models. In the second step, we tried to find a biomarker that can predict efficacy in the optimal population among specific NAFLD phenotypes. The development of biomarkers that can predict treatment response before large-scale clinical trials could increase the success rate and reduce the sample size of trials, thereby reducing the enormous costs. Liver disease is a metabolic disease that is closely related to type 2 diabetes [9,10]. DPP-4 inhibitors, commonly used to treat type 2 diabetes, potentiate their effect on glycemic control by increasing levels of glucagon like peptide-1, a substrate of DPP-4, and are often proposed as a candidate for the treatment of NAFLD. The liver is the highest DPP-4 expressing organ, and serum DPP-4 levels are significantly increased in patients with NAFLD and nonalcoholic steatohepatitis (NASH) [11,12]. In addition, DPP-4 inhibitors can improve steatosis in a mouse model of diet-induced NAFLD [13-15]. However, the mechanism by which antidiabetic drugs directly ameliorate NAFLD/NASH is unclear. Therefore, we aimed to identify the optimal target population or phenotype of patients for DPP-4 inhibitors in NAFLD treatment and to identify a novel biomarker.
Detailed experimental methods are described in the Supplementary Materials.
Six-week-old C57BL/6N wild-type male mice (18ŌĆō20 g) were purchased from Orient Bio Inc. (Orient Animal Laboratory, Seoul, Korea; study I). Six-week-old C57BL/6J wild-type male mice (18ŌĆō20 g) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA; study II, III). The experiment was conducted after a 1-week acclimatization period. The animals were kept in a specific pathogen-free room in which temperature and humidity were maintained at 23┬░C┬▒2┬░C and 60%┬▒10%, respectively, under a 12-hour light/12-hour dark cycle. All experimental procedures were approved by the Hanyang Institutional Animal Care and Use Committee (study I: HY-IACUC-19-0010, study II: HY-IACUC-20-0017, and study III: HY-IACUC-21-0043).
Three diet-induced NAFLD models were used to modify the basket trial. A 60 kcal% high-fat diet (HFD; Research Diet #D12492), high-cholesterol Western diet (WD; Research Diet #D12079B), and methionine choline-deficient diet (MCD; Research Diet # A02082002BR) were purchased from Research Diets Inc. (New Brunswick, NJ, USA). The HFD, WD, and MCD were assigned to each group of 10 animals. The treatment group (n=5) received 200 mg/kg (200 ╬╝L) of evogliptin (Dong-A ST Co., Ltd., Seoul, Korea) dissolved in 0.5% (w/v) methylcellulose administered orally each day for 8 weeks. The control group (n=5) received 200 ╬╝L of 0.5% (w/v) methylcellulose administered orally for 8 weeks. All mice were sacrificed 8 weeks after the first administration of the DPP-4 inhibitor, and their liver tissues and blood were harvested in the laboratory.
After administration of the HFD and WD (6 weeks in the case of MCD) for 16 weeks, all animals were randomized according to the NAFLD activity score (NAS) using a pre-study liver biopsy. The pre-study liver biopsy was performed according to a previous protocol [16]. Zoletil (Virbac Laboratories, Carros, France; 40 mg/kg) and Rompun (Bayer Korea, Seoul, Korea; 5 mg/kg) were used to anesthetize the animals via intraperitoneal injection. After an abdominal midline incision (less than 1 cm), the liver was exposed with a cotton swab and a portion was excised. After surgery, the animals were kept under a heating lamp to keep them warm and treated with tetracycline (87,128; Sigma-Aldrich, Deisenhofen, Germany) for 3 days. NAS evaluation was performed after hematoxylin and eosin staining of the liver tissue, and only animals with biopsy-proven fatty livers (NAS Ōēź3 points) were selected.
A validation study was performed only for the HFD group. After administration of the HFD for 20 weeks, a preliminary liver biopsy was performed. The hepatic insulin-like growth factor binding protein 1 (Igfbp-1) expression was evaluated in a preliminary liver biopsy sample. The control (n=3) and evogliptin (200 mg/kg) treatment groups were randomized according to the NAS and the degree of Igfbp-1 expression. The evogliptin treatment groups were divided into high (n=5) and low (n = 6) Igfbp-1 groups. All mice were sacrificed 1 day after the last dose of the drug.
A developmental study was performed in a group fed an L-amino acid diet with 60 kcal% fat with 0.1% methionine and no added choline (choline-deficient high fat; CDHF). The CDHF (Research Diet #A06071302) was purchased from Research Diets, Inc. The CDHF control and the CDHF+linagliptin groups consisted of 10 and 20 mice, respectively. Linagliptin (Yuhan Corporation, Yongin, Korea). was mixed with the feed at a concentration of 1 mg/kg and administered for 10 weeks. The blood was first collected 14 weeks after the ingestion of the CDHF diet; linagliptin was then administered and blood was collected again 10 weeks later.
If the NAS decreased by more than 1 point at the end of treatment compared to that in the pre-study liver biopsy, the mouse was defined as a responder, whereas a non-responder did not show a reduction in the NAS at the end of the treatment compared to baseline NAS.
At the end of the experiment, mouse serum was harvested and stored at ŌłÆ80┬░C for Luminex multiplex analysis. Multiplex analysis was performed by personnel at Woongbee Meditech Biotechnology Inc. (Seoul, Korea), and serum levels of mouse insulin-like growth factor-1, insulin-like growth factor-binding protein (IGFBP)-1, IGFBP-3, and human IGFBP-1 were determined. Standard curves for each cytokine kit (LXSAMSM-01, LXSAMSM-02, and LXSAHM-01; R&D Systems, Minneapolis, MN, USA) were generated using the reference cytokine concentrations provided by the manufacturer.
Three different NAFLD models (basket study design; HFD, MCD, and WD models) were used, and a pre-study liver biopsy was performed (Fig. 1A). There was no change in the body weight and food intake in the mice fed an HFD or WD, body weight and food intake were lower in those fed the MCD (Fig. 1B). A liver biopsy was performed before the mice received DPP-4 inhibitors, and only animals with a NAS of Ōēź3 were selected (Fig. 1C, D). RNA transcriptome data were analyzed from liver biopsies before DPP-4 inhibitor treatment. Multidimensional scaling data showed different patterns for the three NAFLD models. An analysis conducted with the Database for Annotation, Visualization, and Integrated Discovery (DAVID) program (version 6.8; http://david.abcc.ncifcrf.gov/) indicated that the decreased liver regeneration capacity of MCD compared to HFD was associated with an increase in the Usp-2 gene, which decreased cellular adenosine triphosphate levels [17]. Considering that the MCD did not exhibit insulin sensitivity and had low lipid synthesis, genes that were downregulated with the MCD compared to the HFD were those in the Fos gene family (Ptgs2, Myc, JunB, Socs3), which are related to the immune system and response to cytokines [18]. In mice fed the WD, levels of pro-inflammatory cytokines (Ccr2, Ccl6, tumor necrosis factor) and collagen fibril organization (Col1a1, Col1a2, Col3a1) were increased compared to those fed the HFD. HFD specifically led to an increased expression of genes related to cholesterol synthesis pathways (Mvk, Pmvk, Mvd) compared to WD (Fig. 1E, Supplementary Fig. 1).
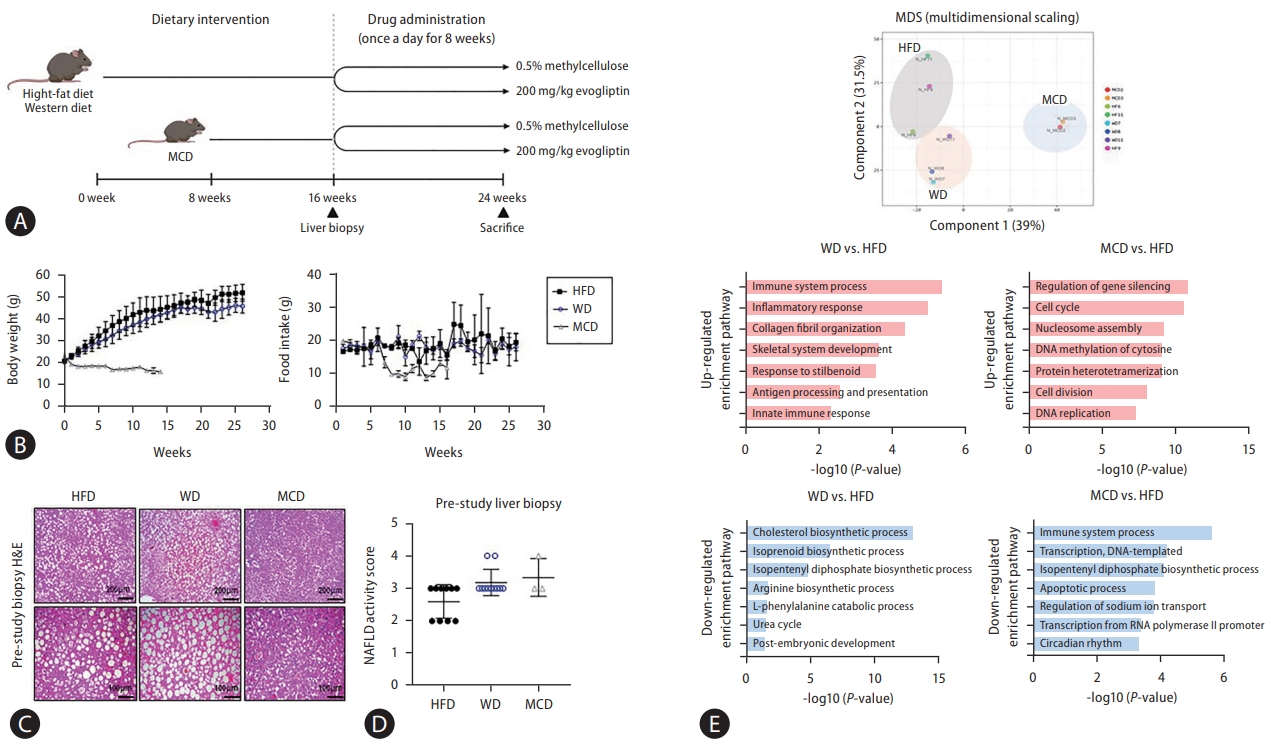
There were no differences in body weight, food intake, and liver weight between control and evogliptin-treated groups in the three NAFLD mouse models (Fig. 2A, B). Hepatic steatosis, hepatocyte ballooning, and NAS were significantly reduced in the HFD. Total NAS and liver inflammation were significantly reduced in the WD, but not in the MCD. The overall response rate to evogliptin was 19.3% in the modified basket NAFLD animal study. The NAS improvement rates were 42%, 25%, and 0% in the HFD, WD, and MCD, respectively (Fig. 2C, D, Table 1). There was no significant difference between the control and evogliptin-treated groups in the three models (Fig. 2E). The quantitative collagen proportional area decreased in the HFD and WD but not in the MCD (Fig. 3A). In addition, messenger RNA (mRNA) expression for fibrosis markers (╬▒-smooth muscle actin [╬▒-SMA], Col1a1, Timp-1) was lower in the evogliptin-treated groups, but not in mice fed the MCD (Fig. 3B). Moreover, mRNA expression of hepatic lipogenesis genes Pparg was decreased in the HFD and WD evogliptin-treated groups compared to the control group. We also found that mRNA expression of very low density lipoprotein secretion markers in the MCD evogliptin-treated group revealed a decrease in Scd-1 compared to the control group but increased expression of ApoB. The expression of the ╬▒-SMA protein decreased in the HFD and WD evogliptin-treated groups compared to the control group (Fig. 3C). The plasma insulin concentration was lower in mice fed the MCD (159.6┬▒4.62) compared with those fed the HFD (181.2┬▒5.92) and WD (174.67┬▒5.06). The receiver operating characteristic curve of the insulin tolerance test was not different in the HFD and WD evogliptin-treated groups compared to the control group (Fig. 3D).
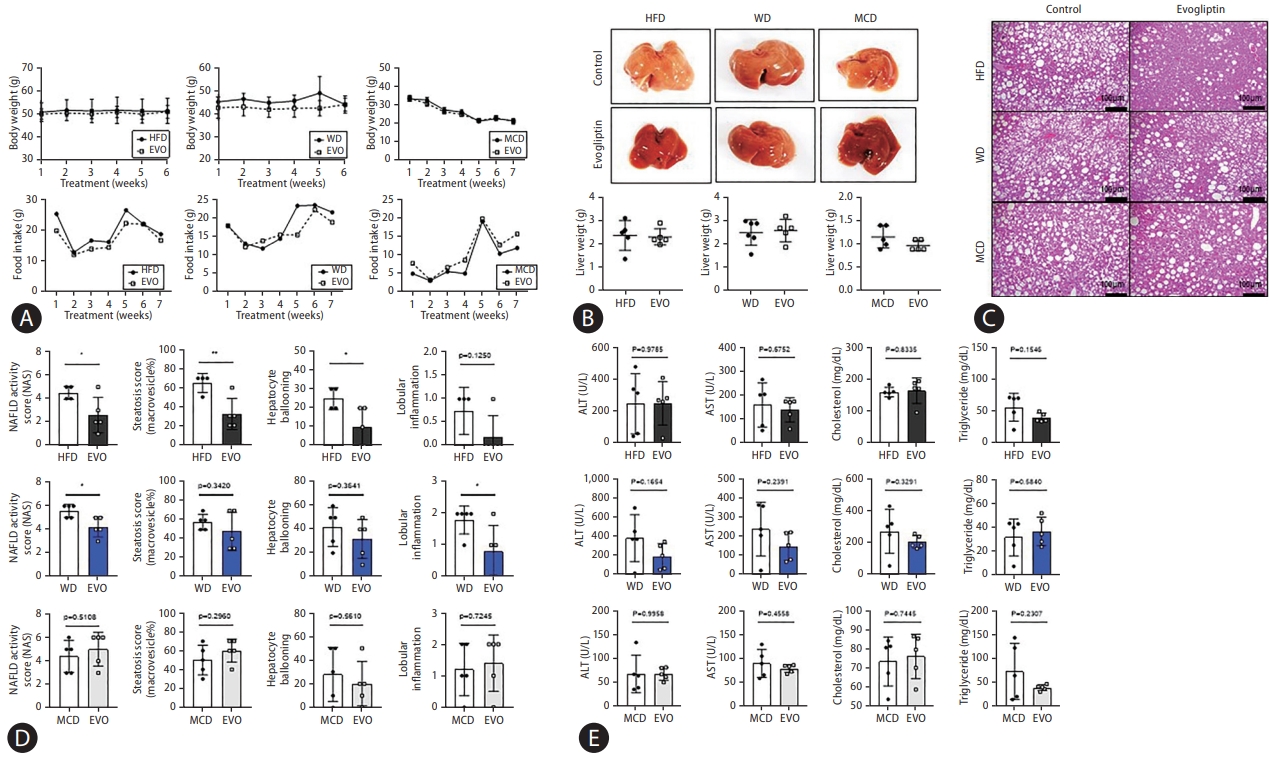
The response and non-response groups were divided according to changes in NAS (Fig. 4A). Responders were defined as those with a NAS that decreased by more than 1 point at the end of treatment compared to the pre-study liver biopsy. Transcriptome analysis was performed for biomarker discovery using pre-study liver biopsy samples from responders and non-responders. Gene set enrichment analysis showed elevated expression of the Igfbp-1-related pathway in the responders compared to that in the non-responders in the pre-study liver biopsy (Fig. 4B, Supplementary Table 1). Moreover, the expression level of Igfbp-1 (log2 fragments per kilobase of transcript per million) was significantly increased with the NAFLD-induced diet (HFD, 7.70; WD, 6.27; MCD, 4.97) compared to the normal chow diet (2.56). Hepatic Igfbp-1 expression was higher in the HFD and responder groups (Fig. 4C, Supplementary Fig. 2).
Hepatic Igfbp-1 expression was compared between responders and non-responders at the end-study liver biopsy. Hepatic Igfbp-1 expression was higher in responders than in non-responders in the pre-study liver biopsy. However, hepatic Igfbp-1 expression was lower in responders than in nonresponders at the end-study liver biopsy (Fig. 4D). The expression of hepatic fibrosis markers was also lower in responders than in non-responders (Fig. 4E).
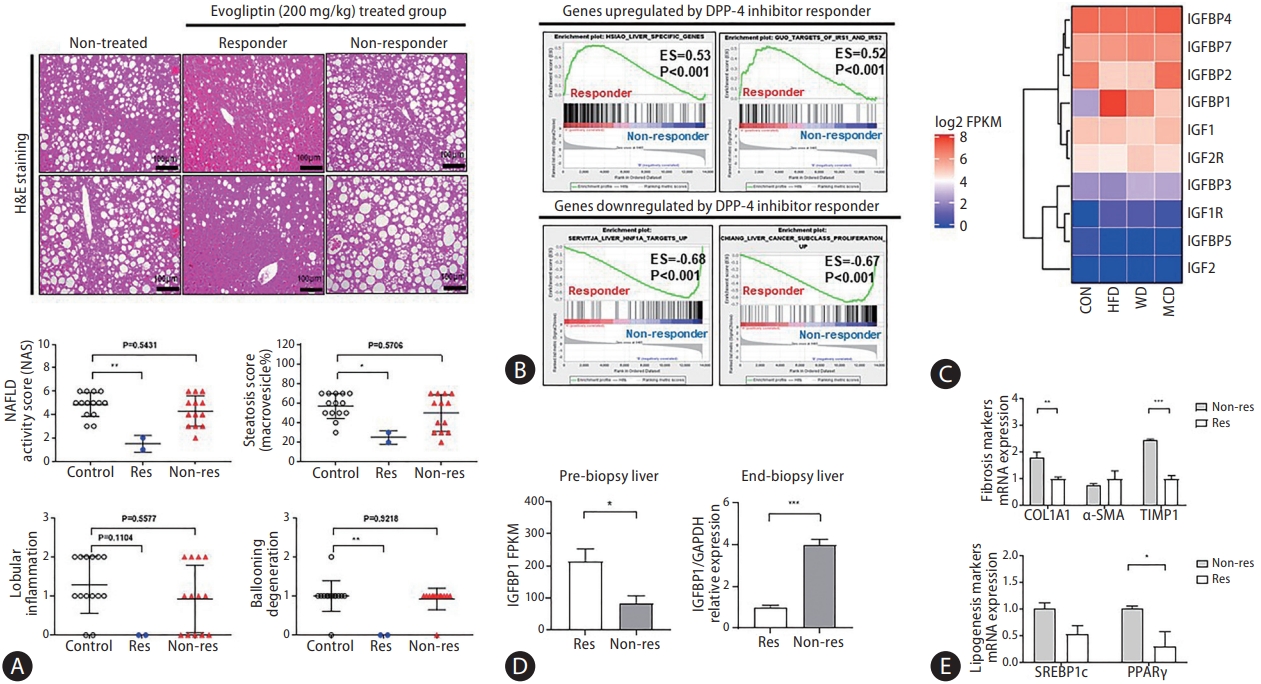
The validation study for DPP-4 inhibitor-specific biomarkers was performed in an additional HFD study. A pre-study liver biopsy was performed at 20 weeks. The HFD was divided into two groups according to hepatic Igfbp-1 expression (high vs. low Igfbp-1) before the evogliptin treatment (Fig. 5A, Supplementary Fig. 3A). Liver and body weights did not differ between the high and low Igfbp-1 groups after evogliptin treatment (Supplementary Fig. 3B). The total NAS was lower in the high Igfbp-1 group than in the low Igfbp-1 group at the end-study liver biopsy (2.8 vs. 5.2, P<0.05) (Fig. 5B, Supplementary Table 2). The levels of hepatic collagen deposition and ╬▒-SMA expression were lower in the high Igfbp-1 group than in the low Igfbp-1 group (Fig. 5C). The levels of the inflammatory marker interleukin-6 receptor were significantly decreased in both low Igfbp-1 (P=0.0278) and high Igfbp-1 (P=0.0013) groups compared with the HFD control group. Igfbp-1 expression was lower in the high Igfbp-1 group (P=0.0368) than in the HFD control group. The serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase were significantly lower in the evogliptin-treated group than in the HFD control group (Supplementary Fig. 3C), but there was no difference according to Igfbp-1 expression level (Supplementary Fig. 3D). In mRNA expression analysis, fibrosis markers ╬▒-SMA, Col1a1, and Timp-1 were significantly lower in the evogliptin-treated group than in the HFD control group (Supplementary Fig. 3E). In addition, mRNA expression for the fibrosis markers ╬▒-SMA and Col1a1 was lower in the high Igfbp-1 group than in the low Igfbp-1 group (Fig. 5D). Additionally, we analyzed the levels of beta-oxidation markers (Acox1, Cpt1a) and lipogenesis markers (Fas, Scd-1). As a result, Fas increased in the high Igfbp-1 group, and Scd-1 was decreased in both the low and high Igfbp-1 groups. Protein expression of FN-1 and p-GSK3╬▓ were also lower in the high Igfbp-1 group than in the low Igfbp-1 group (Fig. 5E). Analysis of the protein expression levels of DPP-4/CD26 revealed decreases in the high Igfbp-1 group compared to the HFD control group (Fig. 5F).
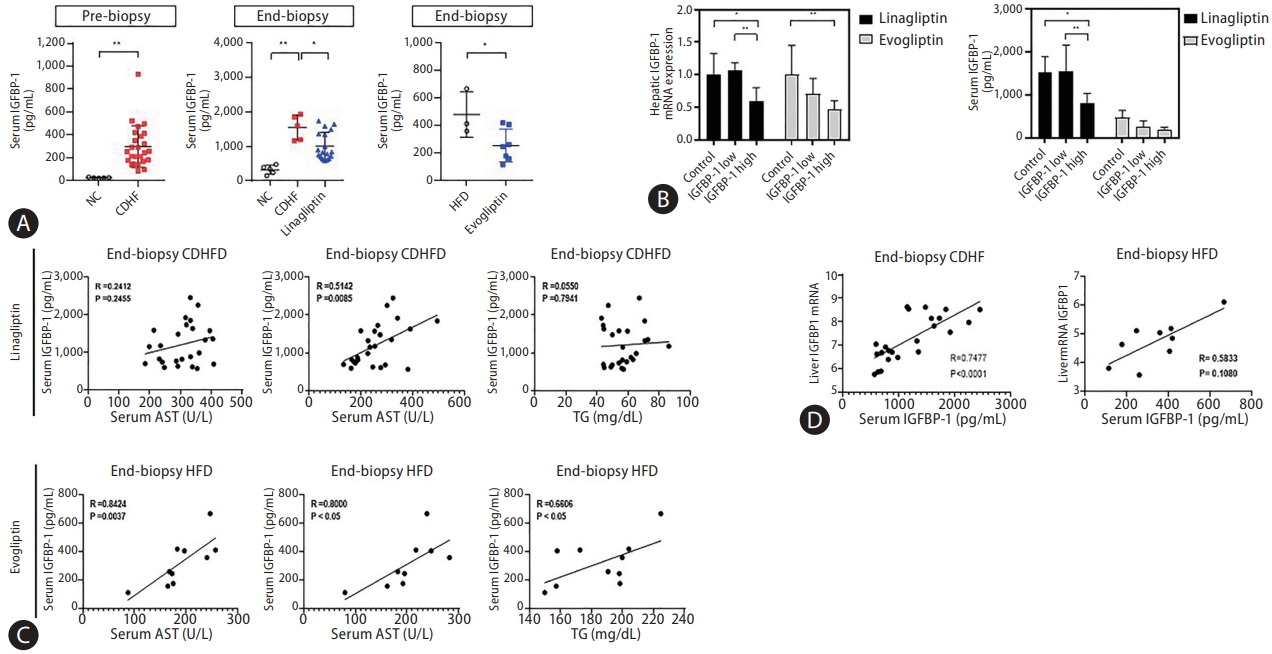
The correlation between the serum IGFBP-1 (sIGFBP-1) concentration and hepatic IGFBP-1 level was evaluated. Levels of sIGFBP-1 increased in the NAFLD patients compared to healthy controls. In addition, sIGFBP-1 increased in the NAFLD mouse model and decreased after treatment with DPP-4 inhibitors (Fig. 6A, Supplementary Fig. 4). Interestingly, hepatic Igfbp-1 expression and sIGFBP-1 concentration decreased after DPP-4 inhibitors treatment only in the high Igfbp-1 group (Fig. 6B). Changes in serum IGFBP-3 (sIGFBP-3) and serum IGF-1 (sIGF-1) levels were not significant in the NAFLD mouse model or after DPP-4 inhibitor treatment (Supplementary Fig. 5A). Levels of sIGFBP-1 were positively correlated with ALT levels before and after DPP-4 inhibitor treatment (Fig. 6C, Supplementary Fig. 5B). Hepatic mRNA Igfbp-1 expression also showed a positive correlation with sIGFBP-1 levels (Fig. 6D). There was no significant difference in the correlation analysis results between liver Igfbp-1 and sIGFBP-3 or sIGF-1 in various DPP-4 inhibitor studies (Supplementary Fig. 5C). In addition, in an analysis of the effect of the DPP-4 inhibitor according to sIGFBP-1 or sIGFBP-3 before administration, NAS was significantly decreased in the high sIGFBP-1 group (Supplementary Table 3). The correlation between blood IGFBP-1 concentration and DPP-4 inhibitors in the NAFLD mouse model was verified through paired and serum analyses before and after drug treatment. The concentration of sIGFBP-1 was confirmed to be positively correlated with the expression of IGFBP-1 in the liver.
The overall response rate to DPP-4 inhibitors was 19.0% in the modified basket NAFLD study. Hepatic Igfbp-1 expression was higher in the responders than in the non-responders; however, after DPP-4 inhibitor treatment, it was lower in the responders. The overall response rate to DPP-4 inhibitors was 87% in patients with high Igfbp-1 levels at the pre-study stage. Thus, high Igfbp-1 levels at the pre-study stage may be a DPP-4 inhibitor-specific biomarker for NAFLD treatment.
The strengths of this study are as follows. First, we modified the basket study design to include an animal model for the first time. The basket trial design was applied to customize treatment in specific cancer patients [19,20]. This new study design was proposed to develop drug-specific biomarkers for various drugs and to discover an optimal target group. To date, the criteria for selecting animal models for preclinical studies of NAFLD have been ambiguous. Our proposed basket NAFLD animal study suggests an optimal target group for NAFLD and demonstrates a more objective efficacy using an unbiased setting. Second, a pre-study liver biopsy was performed before drug administration, and randomization was performed according to histologic severity. We believe that this method provides a high level of evidence on the response to treatment. A previous study reported heterogeneity despite the induction of NAFLD in mice with the same diet and genetic background [16]. Therefore, we tried to obtain more objective results by excluding animals that did not develop fatty liver, as determined in a pre-study liver biopsy, which can stratify the degree of fatty liver in each group. Third, we validated the proposed biomarker by using an additional validation study to verify the predictive performance of the biomarker proposed in the modified basket study.
Currently, there are approximately 11 DPP-4 inhibitors in the global market. Despite slight differences in efficacy, DPP-4 inhibitors can lower blood sugar levels. However, their effectiveness in the treatment of NAFLD has not yet been established [21]. Data on DPP-4 inhibitors in patients with NAFLD are limited. To date, only four clinical studies have been published; these were based on MRI results, and the improvement rate of intrahepatic fatty liver was 8% [22-25]. However, there is no liver histology-based clinical data regarding DPP4 inhibitors in NAFLD. In our study, the mean response rate of DPP-4 inhibitors in the basket NAFLD animal model was 19%. The response rates of the three NAFLD animal models were different for each model (25%, 42%, and 0% in the WD, HFD, and MCD models, respectively). When we applied a new biomarker (IGFBP-1), the response rate of the DPP-4 inhibitor was 83% in the high Igfbp-1 group. Although we demonstrated that the expression of intrahepatic Igfbp-1 showed a good correlation with sIGFBP-1 levels, this study did not suggest a cut-off point for IGFBP-1 that could indicate a good response.
In this study, the high expression of Igfbp-1 in a liver biopsy increased the response rate, and this expression decreased in the liver and serum after the administration of a DPP-4 inhibitor. IGFBP-1 is associated with insulin resistance and predicts the development of type 2 diabetes [26]. Given that DPP-4 inhibitors are used in the management of diabetes, it seems logical that IGFBP-1 may predict the treatment response to DPP-4 inhibitors. Therefore, hepatic Igfbp-1 may be involved in the hepatic insulin signaling pathway. Hepatic Igfbp-1 mRNA expression is inversely associated with glycemia and insulin resistance in patients with NAFLD [27]. Interestingly, the hepatic expression levels of Irs-1, Irs-2 and IGFBPs were higher in the HFD model than in the other diet models. In addition, the response to the DPP-4 inhibitor was also the best in the HFD model in our study.
Our study has some limitations. We confirmed that the treatment response was high when hepatic Igfbp-1 expression was high before treatment. However, there is no clear cutoff for high and low levels of hepatic Igfbp-1. We could not suggest a method for quantifying the expression level of Igfbp-1 in the liver. We observed a positive correlation between sIGFBP-1 levels and Igfbp-1 expression in the liver. However, the correlation was not sufficient to replace the hepatic Igfbp-1 level; therefore, it is difficult to predict treatment response using the sIGFBP-1 level. Additional research is required to generalize the above data to all DPP-4 inhibitors.
In conclusion, the overall response rate to DPP-4 inhibitors was 19% in this basket NAFLD animal model study. The DPP4 inhibitor response was higher in the HFD model than in the other types of NAFLD models, and the response rate increased to 83% in the high Igfbp-1 HFD model. Based on these results, we suggest that the high expression of Igfbp-1 before DPP-4 inhibitor treatment increases the treatment response rate in a NAFLD model.
ACKNOWLEDGMENTS
This study was supported by a research fund from the Research Center of Dong-A ST., Ltd. and Yuhan Research Institute. This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2020R1A2C2009227).
Cartoons in Figures 1A, Supplementary Figure 3A, and graphical abstract were created with BioRender.com.
FOOTNOTES
AuthorsŌĆÖ contributions
D.W.J. designed the study. J.H.O., S.M.L., H.Y.K., and H.L. analyzed the data. E.L.Y., J.H., J.H.P., H.K., and W.K. interpreted the data. J.H.O. performed experiments and wrote the manuscript. D.W.J. is the guarantor of this work, has full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Supplementary┬ĀFigure┬Ā1.
The Database for Annotation, Visualization, and Integrated Discovery (DAVID) functional enrichment analyses of gene expression levels in three types of non-alcoholic fatty liver disease (NAFLD) mouse models. Log2-fold change in gene expression in three types of NAFLD mouse models. MCD, methionine-choline deficient diet; HFD, high-fat diet.
Supplementary┬ĀFigure┬Ā2.
Expression of insulin-like growth factor (IGF)-related genes in three non-alcoholic fatty liver disease (NAFLD) models. Heat map showing gene expression changes of three NAFLD models compared to a normal chow group (log2 fold change, log2FC). IGFBP, insulin-like growth factor-binding protein; ITGB, integrin subunit beta; ITGA, integrin subunit alpha; CCN, cellular communication network factor; ESM, endothelial cell specific molecule; HTRA, high temperature requirement A; CRIM, cysteine rich transmembrane BMP regulator; KAZALD, kazal type serine peptidase inhibitor domain; IGFALS, insulin like growth factor binding protein acid labile subunit; LRP, low density lipoprotein receptor-related protein; HF, high-fat diet; WD, Western diet; MCD, methionine choline-deficient diet; MELTF, melanotransferrin; TMEM, transmembrane protein; AFP, alpha fetoprotein; SPP, secreted phosphoprotein; PAPPA, pappa pregnancy-associated plasma protein; KLK, kallikrein related peptidase; VCAN, versican; TNC, tenascin C; LGALS, lectins, galactoside-binding; MGAT4A, alpha-1,3-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase A; TIMP, tissue inhibitor of matrix metalloproteinase; SERPINA, serpin family A member; GPC, glypican; TLR, toll-like receptors; FLT, fms related receptor tyrosine kinase; IRS, insulin receptor substrate; ATP, adenosine triphosphate; FGF, fibroblast growth factors.
Supplementary┬ĀFigure┬Ā3.
Efficacy of treatment with dipeptidyl peptidase (DPP)-4 inhibitors according to the high-fat diet (HFD) model in the validation study. (A) Schematic diagram for the validation study. Representative images of Hematoxylin and Eosin (H&E) staining of the pre-study biopsy liver tissue from HFD. NAFLD activity scores (NAS) were quantified. (B) Body weight and food intake change over time. (C) Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, and triglyceride levels. (D) Serum analysis results after DPP- 4 inhibitor administration according to the insulin-like growth factor-binding protein (IGFBP)-1 expression level before administration. (E) mRNA expression of liver fibrosis markers and very low density lipoprotein secretion markers. All data are expressed as the mean┬▒standard deviation. NAFLD, non-alcoholic fatty liver disease; NC, normal chow; EVO, evogliptin; TIMP, tissue inhibitor of matrix metalloproteinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ╬▒-SMA, ╬▒-smooth muscle actin. *P=0.01ŌĆō0.05. **P=0.001ŌĆō0.01. ***P<0.001.
Supplementary┬ĀFigure┬Ā4.
Serum insulin-like growth factor-binding protein (IGFBP)-1 value correlates to human liver disease. Comparison of serum IGFBP-1 levels in the healthy control group and the non-alcoholic fatty liver disease (NAFLD) patient group. All data are expressed as the mean┬▒standard deviation. **P=0.001ŌĆō0.01.
Supplementary┬ĀFigure┬Ā5.
Serum insulin-like growth factor-binding protein (IGFBP)-1 value correlates to various dipeptidyl peptidase (DPP)- 4 inhibitor studies but not in IGFBP-3. (A) Comparison of serum IGFBP-3 and insulin-like growth factor (IGF)-I levels in the control and DPP-4 inhibitor groups before and after treatment. (B) Result of correlation analysis between serum IGFBP-1 and alanine aminotransferase (ALT), aspartate aminotransferase (AST), and triglyceride levels in the pre-biopsy study. (C) Result of correlation analysis between liver messenger RNA (mRNA) IGFBP-1 and serum IGF-I, IGFBP-3 levels in various end-biopsy studies. All data are expressed as the mean┬▒standard deviation. NC, normal chow; CDHF, choline-deficient high-fat diet; HFD, high-fat diet. *P=0.01ŌĆō0.05. ***P<0.001.
Supplementary┬ĀTable┬Ā2.
Validation study (study II): summary of the baseline characteristics of the population
Supplementary┬ĀTable┬Ā3.
Development study (study III): summary of the baseline characteristics of the population
Supplementary┬ĀTable┬Ā4.
List of mouse primer sequences used in this study
Supplementary┬ĀTable┬Ā5.
List of primary and secondary antibodies used in this study
Figure┬Ā1.
Pre-study liver biopsy and baseline RNA transcriptome data according to three different non-alcoholic fatty liver disease (NAFLD) models. A biopsy-proven NAFLD model was used to increase the response rate to the drug by performing a liver biopsy before drug administration through various liver disease models. (A) Diagram showing the treatment schedule for the pre-study biopsy. (B) Body weight and food intake change over time. (C) Representative images of hematoxylin and eosin (H&E) staining of pre-study biopsy liver tissue from mice fed various diets (original magnification, ├Ś20). (D) NAFLD activity scores. (E) Multidimensional scaling and Database for Annotation, Visualization, and Integrated Discovery functional enrichment analyses performed for RNA-seq data from liver tissues of mice with various liver diseases. HFD, high-fat diet; MCD, methionine-choline deficient diet; WD, Western diet.
Figure┬Ā2.
Treatment efficacy according to various non-alcoholic fatty liver disease (NAFLD) models. NAFLD was induced in C57BL6 mice by feeding a high-fat diet (HFD) or Western diet (WD) for 16 weeks or a methionine choline deficient (MCD) diet for 6 weeks. Then, 200 mg/kg evogliptin was administered orally, daily for 8 weeks. (A) Changes in body weight and food intake over time. (B) Microscopic view of the livers and liver weight. (C) Representative images of hematoxylin and eosin (original magnification, ├Ś20). (D) NAFLD activity scores (NAS). (E) Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholesterol, and triglyceride levels. All data are expressed as the mean┬▒standard deviation of five mice per group. EVO, evogliptin. *P=0.01ŌĆō0.05. **P=0.001ŌĆō0.01.
Figure┬Ā3.
Dipeptidyl peptidase-4 inhibitor decreased liver fibrosis in mice that were fed a Western diet (WD) or high-fat diet (HFD). (A) Sirius red staining (% area) for 3 mice per group (original magnification, ├Ś10). (B) Messenger RNA (mRNA) expression levels of fibrosis-related markers, very low density lipoprotein secretion markers, and lipogenesis markers. (C) Protein expression levels were related to fibrosis markers, inflammation, and insulin-resistance signaling markers. (D) Insulin resistance test. All data are expressed as the mean┬▒standard deviation. MCD, methionine-choline deficient diet; EVO, evogliptin; ╬▒-SMA, ╬▒-smooth muscle actin; IL, interleukin; AUC, area under the ROC curve. *P=0.01ŌĆō 0.05. **P=0.001ŌĆō0.01. ***P<0.001.

Figure┬Ā4.
Determining a novel biomarker to predict the treatment response and target population. An RNA transcriptome analysis algorithm was used to select biomarkers from the liver tissue obtained by liver biopsy before drug administration. (A) Representative images of Hematoxylin and Eosin (H&E) staining in res (responder) and non-res (non-responder). Non-alcoholic fatty liver disease (NAFLD) activity scores (NAS) were quantified. (B) Gene set enrichment analysis of the responder was compared to that of the non-responder. (C) Heatmap showed the gene expression value (log2 FPKM) in the three NAFLD mice models compared to mice fed normal chow (CON). (D) Comparison of Igfbp-1 messenger RNA (mRNA) expression between responder and non-responder livers. (E) mRNA expression of liver fibrosis markers and lipogenesis markers in responder and non-responder. All data are expressed as the mean┬▒standard deviation. DPP, dipeptidyl peptidase; ES, enrichment score; FRKM, fragments per kilobase of transcript per million; IGFBP, insulin-like growth factor-binding protein; HFD, high-fat diet; WD, Western diet; MCD, methionine-choline deficient diet; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. *P=0.01ŌĆō0.05. **P=0.001ŌĆō0.01. ***P<0.001.
Figure┬Ā5.
Differences in liver disease phenotype according to the difference in IGFBP-1 expression. (A) Hepatic Igfbp-1 messenger RNA (mRNA) expression comparison in pre-study and end-study liver biopsies. (B) Representative images of hematoxylin and eosin (H&E), Sirius red staining, and ╬▒-SMA, IL-6R, and IGFBP-1 IHC staining from end-study liver biopsy. Non-alcoholic fatty liver disease (NAFLD) activity scores (NAS) were quantified. (C) The % area of Sirius red and ╬▒-SMA, IL-6R, and IGFBP-1 staining were quantified. (D) mRNA expression of liver fibrosis markers, lipogenesis markers, and beta-oxidation markers. (E) Western blot analysis in end-study liver biopsy. (F) Hepatic DPP-4 protein expression comparison between low IGFBP-1 and high IGFBP-1 groups compared to HFD control. All data are expressed as the mean┬▒standard deviation. IGFBP, insulin-like growth factor-binding protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HFD, high-fat diet; EVO, evogliptin; ╬▒-SMA, ╬▒-smooth muscle actin; IL, interleukin; IHC, immunohistochemistry; DPP, dipeptidyl peptidase. *P=0.01ŌĆō0.05. **P=0.001ŌĆō 0.01. ***P<0.001.

Figure┬Ā6.
Insulin-like growth factor-binding protein (IGFBP)-1 correlation between the serum and liver in the development study. (A) Comparison of serum IGFBP-1 (sIGFBP-1) levels in various dipeptidyl peptidase (DPP)-4 inhibitor studies. (B) Comparison of liver and sIGFBP-1 expression after administration according to IGFBP-1 expression level before administration in various DPP-4 inhibitor studies. (C) Result of correlation analysis between sIGFBP-1 and alanine aminotransferase (ALT), aspartate aminotransferase (AST), and triglyceride (TG) levels in various DPP-4 inhibitor studies. (D) Correlation analysis between liver and serum IGFBP-1 levels in various DPP-4 inhibitor studies. All data are expressed as the mean┬▒standard deviation. NC, normal chow; CDHF, choline-deficient high-fat diet; HFD, high-fat diet. *P=0.01ŌĆō0.05. **P=0.001ŌĆō0.01.
Table┬Ā1.
Exploration study (study I): summary of the baseline characteristics of the population
Abbreviations
ALT
alanine aminotransferase
CDHF
choline-deficient high fat
DAVID
Database for Annotation
DPP
dipeptidyl peptidase
HFD
high-fat diet
Igfbp-1
insulin-like growth factor binding protein 1
IGFBP
insulin-like growth factor-binding protein
MCD
methionine choline-deficient diet
mRNA
messenger RNA
NAFLD
non-alcoholic fatty liver disease
NAS
NAFLD activity score
NASH
nonalcoholic steatohepatitis
sIGF-1
serum IGF-1
sIGFBP-1
serum IGFBP-1
sIGFBP-3
serum IGFBP-3
WD
Western diet
╬▒-SMA
╬▒-smooth muscle actin
REFERENCES
1. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019;4:389-398.


2. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577-1586.



3. Connolly JJ, Ooka K, Lim JK. Future pharmacotherapy for nonalcoholic steatohepatitis (NASH): review of phase 2 and 3 trials. J Clin Transl Hepatol 2018;6:264-275.



4. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908-922.




5. Eslam M, George J. Genetic insights for drug development in NAFLD. Trends Pharmacol Sci 2019;40:506-516.


6. Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol 2020;73:26-39.


7. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of nonalcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394:2184-2196.

8. Park JJH, Siden E, Zoratti MJ, Dron L, Harari O, Singer J, et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials 2019;20:572.




9. Dharmalingam M, Yamasandhi PG. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J Endocrinol Metab 2018;22:421-428.



10. Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do diabetologists stand? Clin Diabetes Endocrinol 2020;6:9.




11. Mentzel S, Dijkman HB, Van Son JP, Koene RA, Assmann KJ. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem 1996;44:445-461.



12. Barchetta I, Ceccarelli V, Cimini FA, Barone E, Sentinelli F, Coluzzi M, et al. Circulating dipeptidyl peptidase-4 is independently associated with the presence and severity of NAFLD/NASH in individuals with and without obesity and metabolic disease. J Endocrinol Invest 2021;44:979-988.




13. Nakamura K, Fukunishi S, Yokohama K, Ohama H, Tsuchimoto Y, Asai A, et al. A long-lasting dipeptidyl peptidase-4 inhibitor, teneligliptin, as a preventive drug for the development of hepatic steatosis in high-fructose diet-fed ob/ob mice. Int J Mol Med 2017;39:969-983.


14. Kim MK, Chae YN, Ahn GJ, Shin CY, Choi SH, Yang EK, et al. Prevention and treatment effect of evogliptin on hepatic steatosis in high-fat-fed animal models. Arch Pharm Res 2017;40:268-281.



15. Alam S, Ghosh J, Mustafa G, Kamal M, Ahmad N. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: a 1-year randomized control trial. Hepat Med 2018;10:23-31.




16. Chae YJ, Jun DW, Saeed WK, Kang HT, Oh JH, Lee SM, et al. Feasibility and stability of liver biopsy before treatment for preclinical nonalcoholic fatty liver studies. J Korean Med Sci 2019;34:e14.




17. Donthamsetty S, Bhave VS, Mitra MS, Latendresse JR, Mehendale HM. Nonalcoholic fatty liver sensitizes rats to carbon tetrachloride hepatotoxicity. Hepatology 2007;45:391-403.


18. Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A 2004;101:10422-10427.



19. Siu LL, Conley BA, Boerner S, LoRusso PM. Next-generation sequencing to guide clinical trials. Clin Cancer Res 2015;21:4536-4544.




20. Doostparast Torshizi A, Wang K. Next-generation sequencing in drug development: target identification and genetically stratified clinical trials. Drug Discov Today 2018;23:1776-1783.


21. Snyder HS, Sakaan SA, March KL, Siddique O, Cholankeril R, Cummings CD, et al. Non-alcoholic fatty liver disease: a review of anti-diabetic pharmacologic therapies. J Clin Transl Hepatol 2018;6:168-174.



22. Macauley M, Hollingsworth KG, Smith FE, Thelwall PE, Al-Mrabeh A, Schweizer A, et al. Effect of vildagliptin on hepatic steatosis. J Clin Endocrinol Metab 2015;100:1578-1585.



23. Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2016;65:369-376.



24. Deng XL, Ma R, Zhu HX, Zhu J. Short article: a randomized-controlled study of sitagliptin for treating diabetes mellitus complicated by nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2017;29:297-301.


25. Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology 2019;69:2414-2426.




- TOOLS
-
METRICS

- ORCID iDs
-
Dae Won Jun

https://orcid.org/0000-0002-2875-6139Hyunsung Kim

https://orcid.org/0000-0002-8935-7414 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print



