| Clin Mol Hepatol > Volume 28(2); 2022 > Article |
|
ABSTRACT
Hepatitis C virus (HCV) infection is the second most common cause of chronic liver disease in South Korea, with a prevalence ranging from 0.6% to 0.8%, and HCV infection incidence increases with age. The anti-HCV antibody test, which is cheaper than the HCV RNA assay, is widely used to screen for HCV infections; however, the underdiagnosis of HCV is a major barrier to the elimination of HCV infections. Although several risk factors have been associated with HCV infections, including intravenous drug use, blood transfusions, and hemodialysis, most patients with HCV infections present with no identifiable risk factors. Universal screening for HCV in adults has been suggested to improve the detection of HCV infections. We reviewed the cost-effectiveness of HCV screening and the methodologies used to perform screening. Recent studies have suggested that universal HCV screening and treatment using direct-acting antivirals represent cost-effective approaches to the prevention and treatment of HCV infection. However, the optimal timing and frequency of HCV screening remain unclear, and further studies are necessary to determine the best approaches for the elimination of HCV infections.
HCV infections represent a substantial global medical and economic burdens, with an estimated 58 million people living with chronic HCV infections in 2021 worldwide [1]. Although the introduction of HCV screening among blood donors in 1992 has decreased the rate of HCV transmission in South Korea [2], HCV remains the second-most common cause of chronic liver diseases in South Korea [3]. Although the epidemiological transition patterns associated with HCV infection have varied in the past few decades [4], the prevalence of HCV infections in South Korea ranges from 0.6% to 0.78%, and its incidence increases with age [5-8]. The Korea National Health and Nutrition Examination Survey reported that the prevalence of anti-HCV antibodies among Koreans Ōēź10 years old was 0.66%, whereas among those Ōēź20 years old, the prevalence was 0.71% between 2012 and 2016 [5]. In a nationwide epidemiological study, 0.78% of participants tested positive for anti-HCV antibodies after adjustment for age, sex, and area of residence [6]. Additionally, 30.5ŌĆō46.5% of the population with antiHCV antibodies had detectable HCV RNA [5,9]. Risk factors for HCV infections include older age, needle-stick injuries, dental procedures, multiple sexual partners, blood transfusions before 1991, and surgeries [10]. A prospective multicenter cohort study found that drug abuse, needle-stick injuries, blood transfusions before 1995, tattoos, and age were independent risk factors for HCV infections [11].
HCV infections are often asymptomatic, but disease progression over time can result in the development of complications, such as ascites, variceal bleeding, and liver cancer. Symptoms typically only appear during the advanced stages of hepatitis C, at which point the disease-related damage is difficult to reverse, resulting in considerable medical expenses [12]. Chronic hepatitis C (CHC) may progress to cirrhosis over 20ŌĆō30 years, and the annual incidences of hepatocellular carcinoma (HCC) and hepatic decompensation are 1ŌĆō7% and 3ŌĆō6%, respectively, among individuals with CHC [13]. A study using data obtained from the Korean National Health Insurance showed that the total costs (both direct and indirect) associated with hepatitis C in South Korea increased from USD 501.4 million in 2008 to USD 607.8 million in 2011. Data obtained from the Korean National Health Insurance database for 181,768 CHC patients in 2013 revealed an all-cause healthcare cost associated with CHC of USD 997 per patient per year [14]. By contrast, in the USA, the economic burden of CHC exceeds USD 10 billion annually [15], and a study of 34,597 CHC patients older than 18 years reported an all-cause healthcare cost of USD 19,665 per patient per year from 2002 to 2013 [16]. Another study conducted in the USA reported a mean lifetime cost for CHC of approximately USD 64,490 [17]. Differences in healthcare systems and national economies between countries contribute to these cost disparities. Healthcare costs were also found to increase markedly with increasing liver disease severity, and in 2013, the annual per-patient costs were USD 895 for CHC; USD 1,873 for cirrhosis; USD 6,495 for HCC; and USD 67,359 for the first year following liver transplantion [18]. Another study reported an increase in the mean health care cost per month with disease progression from CHC (USD 77┬▒80) to compensate cirrhosis (CC; USD 98┬▒94), decompensated cirrhosis (DC; USD 512┬▒1,115), or HCC (USD 504┬▒717) [19].
Before the introduction of direct-acting antivirals (DAAs) in the mid-2010s, HCV infections were treated using pegylated interferon (IFN) and ribavirin, which were often associated with adverse events, low response rates, and long treatment durations. DAAs enabled physicians to treat patients with HCV infection refractory to IFN/ribavirin therapy. Due to an increased sustained virologic response (SVR), fewer adverse events, and short treatment durations, international guidelines recommend the use of DAA therapy in patients with HCV viremia over other treatment options. The World Health Organization (WHO) previously proposed the elimination of hepatitis B and C viral infections by 2030 which would require reducing the numbers of new infections and mortality by 90% and 65%, respectively. To achieve this goal, many countries, including the USA, Japan, Australia, and Taiwan, have attempted to devise political and administrative strategies to promote HCV elimination. However, barriers to HCV elimination remain. Because HCV-infected patients are generally asymptomatic until progression to advanced cirrhosis or HCC, many patients are unaware of their disease status. According to a telephone survey in South Korea, only 9.1% (91/1,003) of participants reported receiving an HCV test [20]. The most common reasons reported for HCV testing included routine check-ups, physicianŌĆÖs recommendations, and elevated liver enzymes. In this survey, 75.1% of the respondents agreed that an anti-HCV antibody test should be included in the National Health Examination. Lack of awareness regarding HCV infections among healthcare workers may also contribute to delayed diagnosis and treatment. The active screening of asymptomatic individuals is necessary because untreated patients can potentially spread HCV.
The anti-HCV antibody test has limited application in diagnosis of HCV infections, but can serve as a useful screening test due to high sensitivity and specificity (Ōēź 99%) [14]. Universal HCV screening has not been included in the Korean National Health Program due to the low prevalence of HCV infections in South Korea and the lack of validation regarding the cost-effectiveness of universal HCV screening in South Korea. However, global attitudes toward HCV screening are changing, and both the WHO and the USA Centers for Disease Control and Prevention (CDC) recommend the performance of HCV screening regardless of HCV infection prevalence. The CDC recommends HCV screening at least once per lifetime in all adults, except in locations where the prevalence of HCV infection is <0.1% [21].
The methodology and modeling used in various studies to assess the cost-effectiveness of HCV screening in South Korea are described below.
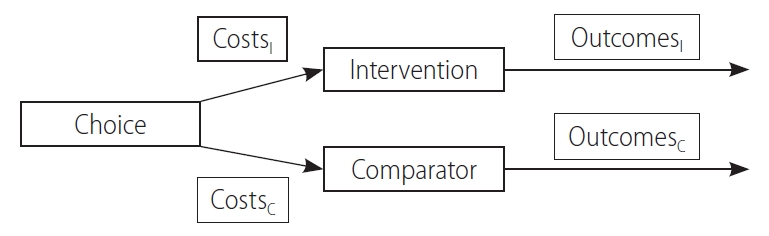
Cost-effectiveness analysis (CEA) compares the economic feasibility of an intervention with that of a comparator (or an alternative) and is typically used interchangeably with economic evaluation (EE). Unlike the cost of illness or outcomes research, CEA is able to assess the costs associated with both inputs and outcomes simultaneously (Fig. 1), and the results are reported as the incremental cost-effectiveness ratio (ICER) [22]. ICER is derived as follows:
where CostsI and OutcomesI are associated with the intervention and CostsC and OutcomesC are associated with the comparator.
Four types of EE have been established, distinguished by the outcome measurement methods: cost-minimization analysis (CMA), cost-benefit analysis (CBA), CEA, and cost-utility analysis (CUA) [22]. CMA can be performed when the outcome is equivalent between two alternatives, with the alternative with the lower input cost regarded as being more economical. CBA can be used for outcomes are expressed in monetary units. Although CBA can be used. CBA has been criticized due to the ethical and methodological issues associated with converting health outcomes into monetary values, this type of analysis can be useful for assessing the performance of large-scale health care programs [23]. In CEA, the outcomes are estimated using natural units. For example, when assessing the outcomes of CHC treatments, the number of HCC cases averted or life-years gained (LYG) can be used to perform CEA. Thus, CEA is preferred by clinicians because the results of CEA are readily comprehensible for application to clinical practice [24]. Numerous government health care agencies recommend performing CUA, which measures outcomes in quality-adjusted life-years (QALYs), a combination of quantity of life (i.e., LYG) and quality of life (QoL, for which death and perfect health are represented by values of 0 and 1, respectively) [25]. For example, if one patient lives for 1 year with perfect health (quantity of life ├Ś QoL = 1 ├Ś 1), but another patient lives for 2 years with a value of 0.5 for QoL (2 ├Ś 0.5), the result is 1 QALY for both cases. Because outcomes are represented by a single measure (i.e., QALY), CUA can be used to compared different types of interventions (i.e., treatments for CHC vs. anticancer agents). Therefore, government agencies recommend the performance of CUA to determine optimal treatment strategies.
The performance of CEA requires data regarding the comparative effectiveness, costs, and utility weights for an intervention and a comparator. Meta-analysis and systematic review provide the highest level of evidence for the comparative effectiveness of two alternatives. Costs are commonly estimated from real-world data, such as electronic medical records or insurance claims. However, cost estimates vary depending on the perspective of the estimators, as payers, the health care system, and overall society are likely to evaluate different variables. For example, from the payerŌĆÖs perspective, costs related to productivity are not relevant, whereas these indirect costs should be included in cost estimates performed from a societal perspective. Utility values are measured directly among cohorts of patients or the general public using validated QoL assessment tools (e.g., EQ-5D or SF-36) or extracted from previous studies.
Most CEA studies use decision-analytic models, which are constructed to reflect relevant evidence, link intermediate and final endpoints, extrapolate long-term outcomes, and aid decision-making in real-world settings [22]. Decision trees and Markov models are two commonly used model types. The decision tree model is commonly employed to evaluate a discrete event within a short period, whereas the Markov model is used to evaluate long-term or chronic illnesses that persist until death. These models consider several health states and should be simple while simultaneously reflecting the natural history of the disease [26]. Assume that the Markov model shown in Supplementary Figure 1 was constructed to perform a CEA or two CHC treatment alternatives (a new medicine, I vs. an existing medicine, C). The hypothetical model includes seven health states, each represented as bubbles: CHC, SVR, CC, DC, HCC, liver transplantation, and death. The CEA was performed by constructing a hypothetical cohort of 1,000 patients that entered the CHC state. Based on this model, when the cycle length is set to 1 year, 70% (transition probability: 0.7) of the starting cohort will progress to SVR, 7% will transition to CC, and 3% will transition to HCC after 1 year using the new medicine. With repeated analyses, the distribution of the cohort changes according to the transition probability. After cycle 1,200 patients remain in the CHC state (1,000 ├Ś 0.2), whereas after cycle 2, only 40 patients (200 ├Ś 0.2) remain in the CHC state (Supplementary Fig. 1). This process can be repeated for all health states via transition probabilities until the end of the time horizon (analysis period), after which the costs and outcomes are calculated according to the distribution of the cohort associated with each health state.
ICER is calculated from CEA or CUA and is defined as the difference in costs divided by the difference in outcomes between two alternatives. ICER indicates the costs required to gain an additional outcome (number of cases averted or QALY), when using the intervention compared with the comparator. Although the acceptability of the additional cost depends on the individual standards, 1 ├Ś gross domestic product (GDP) is generally considered to be the threshold of willingness to pay (WTP) for 1 QALY.
According to prior studies performed in South Korea, the performance of one-time HCV screening and treatment is highly cost-effective, and significantly reducing the morbidity and mortality rates associated with hepatitis C [27,28]. Table 1 summarizes the current research examining the cost-effectiveness of CHC screening and treatment in Korea.
Kim et al. [27] investigated the cost-effectiveness of a one-time HCV screening and treatment program among individuals 40ŌĆō70 years of age using a Markov model in conjunction with a screening and treatment decision tree model. Patients were divided into cohorts according to age: 40ŌĆō49, 50ŌĆō59, and 60ŌĆō69 years, and the prevalence of infection was estimated to be 0.60%, 0.80%, and 1.53%, respectively, for each age group. An estimated 71.7% of individuals were screened, and 39.4% of patients were treated with DAA over 5 years. Screening resulted in the detection of 43,635 new cases across all cohorts. The model predicted that 17,193 patients would require DAA treatments after screening (40ŌĆō49 years: 31.0%, 50ŌĆō59 years: 30.5%, and 60ŌĆō69 years: 38.4%). For screening, the HCV antibody test cost USD 3.49; the HCV quantitative RNA test cost USD 147.33; and the ultrasound cost USD 61.43. The estimated medical costs for the treatment of different disease stages were USD 972.73 for CHC; USD 1,238.02 for CC; USD 6,468.01 for DC; and USD 6,366.94 for HCC. Predicted ICER values ranged from USD 5,714 to USD 8,889 per QALY gained for all patients. Screening and treatment were expected to be highly cost-effective across all patients aged 40ŌĆō69 years, based upon a WTP threshold of USD 27,512. Incremental costs associated with screening and treatment ranged from USD 156.47 to USD 181.85 million. An important finding of this study was that anti-HCV antibody testing was the most cost-effective for individuals aged 40ŌĆō49 years, which is likely due to reduced disease progression associated with the early diagnosis and treatment of HCV in younger individuals, resulting in lower overall lifetime costs.
Kim et al. [28] also investigated the cost-effectiveness of screening and DAA treatment among individuals aged 40ŌĆō65 years. The prevalence of HCV infections was estimated to be 0.38ŌĆō0.53% among individuals aged 40ŌĆō49 years, 0.63ŌĆō0.91% among individuals aged 50ŌĆō59 years, and 0.80ŌĆō1.32% among individuals aged 60ŌĆō69 years. The cost for the HCV antibody test was USD 3.40, whereas the HCV quantitative RNA test cost USD 81.50. The annual healthcare costs were estimated at USD 744.40 for CHC; USD 947.80 for CC; USD 6,113.40 for DC; and USD 6,017.60 for HCC. Screening an estimated 14,103,806 South Koreans identified 82,394 individuals with anti-HCV positivity, among which 38,313 presented with HCV RNA positivity, and 31,608 were diagnosed with CHC. Finally, 20,134 individuals were treated with DAA over 3 years. Screening and treatment increased QALYs by 0.0015 at the cost of USD 11.27 (ICER: USD 7,435 per QALY gained). The probability that this screening strategy would be cost-effective was assessed as 98.8% at a WTP of USD 27,205, and this strategy was predicted to prevent 32 HCV-related deaths, 19 cases of HCC, and 15 cases of DC per 100,000 screened individuals. The assessment of low ICER values, despite the low prevalence of HCV in South Korea, indicates that the cost of HCV screening and treatment is very low. Therefore, a one-time HCV screening and DAA treatment program is likely to be highly cost-effective for reducing HCV-related morbidity and mortality. However, non-medical costs (e.g., lost working days) that may further increase the cost-effectiveness of screening strategies were not investigated.
Two studies have previously investigated whether a ŌĆ£screen allŌĆØ strategy would be cost-effective compared with no screening; however, these studies did not include new DAA regimens, such as ledipasvir/sofosbuvir (LDV/SOF) for genotype (GT) 2 patients, which has been a reimbursed treatment in South Korea starting in June 2019. To reflect changes in treatment, Kim et al. [9] investigated the cost-effectiveness of increased screening with subsequent DAA treatment for all CHC patients Ōēź40 years (screening and DAA treatments were offered again at 65 years if the participant initially refused screening prior to 65 years compared with the current practice of screening only high-risk patients). The prevalence of HCV infections was estimated at 0.38% among individuals aged 40ŌĆō49 years, 0.63% among individuals aged 50ŌĆō59 years, 1.08% among individuals aged 60ŌĆō69 years, and 1.64% among individuals aged Ōēź70 years. The HCV antibody test cost USD 3.14; the HCV quantitative RNA test cost USD 31.70; and ultrasound exams cost USD 125.13. The annual costs for DC were estimated at USD 6,161.70, whereas HCC was estimated at USD 6,065.40. The standard high-risk screening strategy led to the screening of 2,546,832 patients, resulting in the identification of 6,539 HCV RNA positive patients and the DAA treatment of 4,165 patients. A screen-all-individuals-once scenario led to the screening of 15,818,833 patients, resulting in the identification of 40,614 HCV RNA-positive patients and the DAA treatment of 25,871 patients. A screen-all-individuals-twice scenario led to the screening of 4,429,273 additional patients after the age of 65 years. Using a Markov disease progression model, the screen-all-individuals-twice scenario led to the lowest rates of advanced liver disease compared with the screen-all-individuals-once and high-risk-only screening approaches. A screen-all-individuals-once strategy increased QALYs by 49,612 compared with the high-risk-only screening strategy, whereas a screen-all-individuals-twice strategy increased QALYs by an additional 5,075. Using the same LDV/SOF treatment for GT1 and 2, the ICER values for the screen-all-individuals-once and screen-all-individuals-twice strategies were USD 4,535.96 and USD 4,636.33, respectively, compared with the high-risk-only screening strategy. When screen-all-individuals-twice was compared with screen-all-individuals-once, the ICER value for the screen-all-individuals-twice strategy was USD 3,558.18. Thus, the authors of this study concluded that screening all individuals twice, followed by treatment as necessary, would be more cost-effective than the current high-risk-only screening approach.
Another study demonstrated the necessity of increased National Health Insurance coverage for hepatitis C screening and DAA treatment [29]. The estimated prevalence rate of hepatitis C antibody in South Korea is 0.7%, and the costs for the HCV antibody and HCV quantitative RNA tests were reported to be USD 20 and USD 150, respectively. Estimated annual treatment costs were USD 10,000 for CHC; USD 2,064 for CC; USD 6,146 for DC; and USD 8,000 for HCC. This study estimated that in 2016, approximately 46% of South Koreans with chronic HCV infections and 16% with HCV antibodies received DAA treatment. This study used a compartmental age-sex structured model of HCV progression to analyze the cost-effectiveness of increased HCV screening and treatment with DAAs. The policy scenarios that were analyzed in the study included status quo, population screening starting at age 60 years, population screening starting at age 40 years, and population screening starting at age 20 years. All alternative strategies were found to be cost-effective compared with no treatment for preventing infections. Increased screenings among populations aged Ōēź60 years and those aged Ōēź40 were estimated to avoid 15,231 and 17,374 HCV infections, respectively, in addition to preventing 5,310 and 5,798 deaths, respectively, for the period from 2017 to 2050.
Approximately 2.7 million people in the USA have CHC [30]. Among HCV-infected persons, 81% were born between 1945 and 1965. In the USA, high-risk groups (i.e., intravenous drug abusers, patients who received a blood transfusion before 1992, hemodialysis patients, inmates, babies of HCV-infected mothers, and individuals with tattoos) and birth cohorts born from 1945 through 1965 (cohorts with a high prevalence of HCV infection) are screened and treated for HCV [31]. When a birth cohort was screened for treatment with IFN/ribavirin, the ICER value was USD 15,700 QALY compared with the high-risk group. When pegylated IFN/ribavirin/DAA therapy was used, the ICER value was USD 35,700/QALY; therefore, screening and treatment among birth cohorts were reported as cost-effective, and the CDC recommends screening all individuals born between 1945 and 1965 [32]. Because treatment outcomes have significantly improved with the introduction of DAAs, in 2020, the CDC recommended that all individuals aged Ōēź18 years should undergo at least one screening test during their lifetimes [33]. Recently, Eckman et al. [34] analyzed the cost-effectiveness of universal one-time screening for HCV infection in the USA in the era of pan-genotypic DAA therapy, compared with the current standard of birth cohort screening strategy. Using the Markov state transition model, universal one-time screening of the general USA population, assuming a prevalence of HCV antibody greater than 0.07%, resulted in a reduced cost equal to USD 50,000/QALY than the no screening strategy. The model also showed that, compared with one-time birth cohort screening, universal one-time screening and treatment with pangenotypic DAAs cost USD 11,378/QALY increase. This study highlighted the importance of HCV screening among young persons in the USA.
HCV infection is the leading cause of cirrhosis and liver cancer in Japan, where approximately 2 million individuals were estimated to be living with HCV infection in the year 2000 [35]. According to a cost-effectiveness study based on the national HCV screening program, the rates of HCV infections among the general population and among high-risk groups were 0.36% and 0.81%, respectively [36]. Screening for HCV is reportedly cost-effective in both high-risk groups and among the general population (USD 749 to USD 2,297 and USD 848 to USD 4,825, respectively). Due to the high prevalence of HCV infection, nationwide HCV screening was initiated in Japan in 2002. A recent study compared the cost-effectiveness of screening plus IFN-free therapy, no screening, and screening plus IFN-based therapy [37]. The base-case model involved screening all Japanese individuals aged 40ŌĆō89 years. Screening plus IFN-free therapy was more cost-effective than no screening or screening plus IFN-based therapy under a WTP of USD 45,163 per QALY gained in the base-case model, with ICER values of USD 10,157/QALY relative to no screening and USD 9,802/QALY relative to screening plus IFN-based therapy. Importantly, in the age subgroup analysis, ICER values were lower for the younger population (Table 2). Except among the population aged 85 years and older, screening plus IFN-free therapy was the most cost-effective option under the WTP setting. These results suggest that population-based HCV screening of all adults aged below 85 years in Japan represents a reasonable option.
According to a systematic review performed in Europe, the cost of prolonging survival by 1 year through HCV screening ranged between USD 2,856 and USD 17,520 [38], suggesting that screening is cost-effective only in areas with a high prevalence of HCV infection. Unlike the USA and Japan, the prevalence of HCV infection in England is relatively low, with only 143,000 people estimated to be living with HCV infection in 2015 [39]. Another study evaluated the cost-effectiveness of a one-time HCV screening intervention for individuals born between 1950 and 1979, as part of a National Health Service health check [40]. The base-case ICER values ranged from USD 10,408 to USD 33,411, with the lowest ICER observed for people born between 1970 and 1974 and the highest ICER observed for people born between 1950 and 1954. Thus, birth cohort screening in England is likely to be the most cost-effective option for younger birth cohorts; however, whether HCV screening will be cost-effective for other birth cohorts remains uncertain.
France has one of the most extensive HCV screening programs worldwide. A health economic study from France evaluated the cost-effectiveness of five screening scenarios [41]: S1, the current strategy targeting at-risk populations; S2, S1 plus all men aged 18ŌĆō59 years; S3, S1 plus all individuals aged 40ŌĆō59 years; S4, S1 plus all individuals aged 40ŌĆō80 years; and S5, all individuals aged 18ŌĆō80 years (universal screening). Universal screening was found to be more effective and cost-effective (USD 36,446/QALY) than targeting individuals aged 40ŌĆō80 years. However, this strategy is only cost-effective if treatment is initiated during an early stage of infection but not if treatment is started after advanced stages of fibrosis has developed.
The prevalence of HCV seroprevalence in Canada is estimated to be 0.3ŌĆō0.9%. Despite being neighboring countries, the epidemiology of HCV and the health care system in Canada differ from those in the USA. A Canadian study analyzed the cost-effectiveness of one-time HCV screening among individuals aged 25ŌĆō64 and those aged 45ŌĆō64 years [42]. The ICER values ranged from USD 34,359 to USD 44,034 per QALY gained, compared with no screening, depending on the age group screened and the antiviral therapy administered. The authors concluded that a one-time program to screen for and treat HCV infection in Canada, targeted at birth cohort populations (25ŌĆō64 years of age or 45ŌĆō64 years of age), is likely to be cost-effective and suggested that increased treatment success rates would further enhance the cost-effectiveness of all HCV screening programs.
The Global Health Sector Strategy on Viral Hepatitis (2016ŌĆō2021), published by the WHO, calls for all countries to establish firm targets for the elimination of viral hepatitis [43]. Successful HCV elimination requires the establishment and maintenance of all steps in the care cascade, starting with decrease awareness and continuing through treatment. National screening is likely the most important of these steps, and national health policies should establish HCV screening programs to reduce the HCV-related disease burden.
HCV screening should be performed as part of routine screening among individuals with progressive liver disease starting at the age of 40 years. In a 2019 Korean survey of the general public, more than 75% of respondents indicated their belief that screening for HCV is necessary [20]. Because of its low prevalence, HCV screening has not been included in national screening programs. However, the findings across multiple studies highlight the importance of HCV screening, supporting its inclusion in national health policy.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Supplementary┬ĀFigure┬Ā1.
An example of a Markov model for cost-effectiveness analysis of treatments in patients with chronic hepatitis C. This model includes seven health states, each represented by a bubble, reflecting the natural history of chronic hepatitis C. The numbers associated with each error represent the probabilities of transitioning from one health state to another in each cycle. For example, if the cycle length is set to 1 year, the probability of 0.7 (bold, associated with the arrows from chronic hepatitis C to sustained virological response) indicates that 70 out of 100 patients in with chronic hepatitis C will transition to a sustained virological response during the 1-year period.
Figure┬Ā1.
The concept of cost-effectiveness analysis. Cost-effectiveness analyses simultaneously assess both costs and outcomes of an intervention and a comparator. Subscripted I refers to the intervention variables, whereas subscripted C refers to the comparator variables. Reused from Drummond et al. [22]
Table┬Ā1.
Cost-effectiveness studies of screening and treatment for hepatitis C virus infection in Korea
| Study | Screening scenario | Analysis model | Type of outcome | WTP threshold | Perspective | Results | ICER |
|---|---|---|---|---|---|---|---|
| Kim et al. [9] (2020) | 1) Screen all individuals twice (at over 40 years and 65 years of age) | Markov model | Total population costs, and total population QALYs | 3├ŚGDP ($9,293.52/QALY) | Healthcare system (considering only direct medical costs) | Screening all twice followed by LDV/SOF treatments was cost-effective compared with current high risk only screening | 1) $4,535.96 |
| 2) Screen all individuals once (at over 40 years of age) | 2) $4,636.33 | ||||||
| 3) High risk only | 3) REF | ||||||
| Kim et al. [29] (2019) | 1) No treatment | Compartmental age-sex structured model | - | - | - | The expansion of DAA coverage by National Health Insurance and scale-up of hepatitis C screening and treatment with DAAs were cost-effective | 1) REF |
| 2) Status quo | 2) $101,208 | ||||||
| 3) Screening population older than 60 years | 3) $111,770 | ||||||
| 4) Screening population older than 40 years | 4) $107,909 | ||||||
| 5) Screening population older than 20 years | 5) $229,604 | ||||||
| Kim et al. [28] (2019) | 1) No screening | Markov model | Costs and QALY | $27,205 | Healthcare system (considering only direct medical costs) | A one-time HCV screening and DAA treatment of a Korean population aged 40ŌĆō65 years was highly cost-effective | 1) REF |
| 2) Screening once (ages 40ŌĆō65 years) and DAA treatment | 2) $7,435 | ||||||
| Kim et al. [27] (2017) | 1) No screening | Markov model | Costs and QALY | 3├ŚGDP (GLE/PIB $89,041/QALY; LDV/SOF $92,533/QALY) | Korean national payer | One-time HCV screening and treatment in South Korean people aged 40ŌĆō70 years was highly cost-effective | 1) REF |
| 2) One-time screening (ages 40ŌĆō49 years) | 2) $5,385.36 | ||||||
| 3) One-time screening (ages 50ŌĆō59 years) | 3) $6,451.44 | ||||||
| 4) One-time screening (ages 60ŌĆō69 years) | 4) $8,380.46 |
Table┬Ā2.
Cost-effectiveness studies of screening and treatment for hepatitis C in other countries
| Study | Screening scenario | Analysis model | Results | ICER |
|---|---|---|---|---|
| Eckman et al. [34] (2019; USA) | Screen all once (over 18 years) | Markov model | Universal screening was cost-effective compared with birth cohort screening when antibody positivity was greater than 0.07% | Compared with birth cohort screening, universal 1-time screening and treatment cost $11,378/QALY gained |
| Birth-cohort screening (born from 1945 through 1965) | ||||
| Nagai et al. [37] (2020; Japan) | Population aged 45 years | Markov model | Screening followed by IFN-free DAA therapy was cost-effective in all age subpopulations, except for the population aged 85 years, when willingness to pay was $45.163 per QALY | $1,736 |
| Population aged 55 years | $3.13 | |||
| Population aged 65 years | $6,718 | |||
| Population aged 75 years | $18,580 | |||
| Population aged 85 years | $65,199 | |||
| Williams et al. [40] (2019; UK) | Born from 1950 through 1954 | Markov model | Birth cohort screening is likely to be cost-effective for younger birth cohorts | $33,411 |
| Born from 1955 through 1959 | $21,243 | |||
| Born from 1960 through 1964 | $14,415 | |||
| Born from 1965 through 1969 | $10,990 | |||
| Born from 1970 through 1974 | $10,458 | |||
| Born from 1975 through 1979 | $11,207 | |||
| Wong et al. [42] (2015; Canada) | No screening | Markov model | A selective one-time HCV screening program for people 25ŌĆō64 or 45ŌĆō64 years of age in Canada would likely be costeffective | REF |
| One-time screening (age 25ŌĆō64 years) | $34,359 | |||
| One-time screening (age 45ŌĆō64 years) | $44,034 | |||
| Deuffic-Burban et al. [41] (2018; France) | Screening risk population | Markov model | Universal screening is the most effective screening strategy for HCV | REF |
| One-time screening (age 18ŌĆō59 years) | Dominated | |||
| One-time screening (age 40ŌĆō59 years) | Dominated | |||
| One-time screening (age 40ŌĆō80 years) | $43,829 | |||
| Universal screening (age 18ŌĆō80 years) | $17,520 |
Abbreviations
CBA
cost-benefit analysis
CC
compensate cirrhosis
CDC
Centers for Disease Control and Prevention
CEA
cost-effectiveness analysis
CHC
chronic hepatitis C
CMA
cost-minimization analysis
CUA
cost-utility analysis
DAAs
direct-acting antivirals
DC
decompensated cirrhosis
EE
economic evaluation
GDP
gross domestic product
GT
genotype
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
ICER
incremental cost-effectiveness ratio
IFN
interferon
LDV/SOF
ledipasvir/sofosbuvir
LYG
life-years gained
QALYs
quality-adjusted life-years
QoL
quality of life
SVR
sustained virologic response
WHO
World Health Organization
WTP
willingness to pay
REFERENCES
1. World Health Organization (WHO). Progress report on HIV, viral hepatitis and sexually transmitted infections 2019: accountability for the global health sector strategies, 2016ŌĆō2021. Geneva: WHO; 2019.
2. Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, et al. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2020;5:167-228.



3. Lee SS, Byoun YS, Jeong SH, Kim YM, Gil H, Min BY, et al. Type and cause of liver disease in Korea: single-center experience, 2005-2010. Clin Mol Hepatol 2012;18:309-315.



4. Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol 2019;16:57-73.


5. Kim KA, Lee JS. Prevalence, awareness, and treatment of hepatitis C virus infection in South Korea: evidence from the Korea National Health and Nutrition Examination Survey. Gut Liver 2020;14:644-651.



6. Kim DY, Kim IH, Jeong SH, Cho YK, Lee JH, Jin YJ, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int 2013;33:586-594.


7. Kim BK, Jang ES, Kim JH, Park SY, Ahn SV, Kim HJ, et al. Current status of and strategies for hepatitis C control in South Korea. Clin Mol Hepatol 2017;23:212-218.



8. Jang ES, Ki M, Choi HY, Kim KA, Jeong SH; Korean Hepatitis Epidemiology Study Group. The change in the nationwide seroprevalence of hepatitis C virus and the status of linkage to care in South Korea from 2009 to 2015. Hepatol Int 2019;13:599-608.


9. Kim DY, Wong G, Lee J, Kim MH, Smith N, Blissett R, et al. Cost-effectiveness of increased screening and treatment of chronic hepatitis C in Korea. Curr Med Res Opin 2020;36:993-1002.


10. Kim JY, Cho J, Hwang SH, Kil H, Bae SH, Kim YS, et al. Behavioral and healthcare-associated risk factors for chronic hepatitis C virus infection in Korea. J Korean Med Sci 2012;27:1371-1377.



11. Seong MH, Kil H, Kim YS, Bae SH, Lee YJ, Lee HC, et al. Clinical and epidemiological features of hepatitis C virus infection in South Korea: a prospective, multicenter cohort study. J Med Virol 2013;85:1724-1733.


12. El Khoury AC, Klimack WK, Wallace C, Razavi H. Economic burden of hepatitis C-associated diseases in the United States. J Viral Hepat 2012;19:153-160.


14. Alborino F, Burighel A, Tiller FW, van Helden J, Gabriel C, Raineri A, et al. Multicenter evaluation of a fully automated third-generation anti-HCV antibody screening test with excellent sensitivity and specificity. Med Microbiol Immunol 2011;200:77-83.


15. Stepanova M, Younossi ZM. Economic burden of hepatitis C infection. Clin Liver Dis 2017;21:579-594.


16. McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm 2011;17:531-546.


17. Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 2013;57:2164-2170.



18. Ki M, Choi HY, Kim KA, Jang ES, Jeong SH. Healthcare costs for chronic hepatitis C in South Korea from 2009 to 2013: an analysis of the national health insurance claimsŌĆÖ data. Gut Liver 2017;11:835-842.



19. Kim DY, Yoon KT, Kim W, Lee JI, Hong SH, Lee D, et al. Estimation of direct medical cost related to the management of chronic hepatitis C and its complications in South Korea. Medicine (Baltimore) 2016;95:e3896.



20. Choi GH, Jang ES, Kim JW, Jeong SH. A survey of the knowledge of and testing rate for hepatitis C in the general population in South Korea. Gut Liver 2020;14:808-816.



21. Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC recommendations for hepatitis C screening among adults - United States, 2020. MMWR Recomm Rep 2020;69:1-17.



22. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015.
23. McIntosh E, Donaldson C, Ryan M. Recent advances in the methods of cost-benefit analysis in healthcare. Matching the art to the science. Pharmacoeconomics 1999;15:357-367.


25. Torrance GW, Feeny D. Utilities and quality-adjusted life years. Int J Technol Assess Health Care 1989;5:559-575.


26. Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13:397-409.


27. Kim DY, Han KH, Jun B, Kim TH, Park S, Ward T, et al. Estimating the cost-effectiveness of one-time screening and treatment for hepatitis C in Korea. PLoS One 2017;12:e0167770.



28. Kim KA, Chung W, Choi HY, Ki M, Jang ES, Jeong SH. Cost-effectiveness and health-related outcomes of screening for hepatitis C in Korean population. Liver Int 2019;39:60-69.


29. Kim J, Haacker M, Keshavjee S, Atun R. Cost-effectiveness of scaling up of hepatitis C screening and treatment: a modelling study in South Korea. BMJ Glob Health 2019;4:e001441.



30. Centers for Disease Control and Prevention (CDC). Locations and reasons for initial testing for hepatitis C infection--chronic hepatitis cohort study, United States, 2006-2010. MMWR Morb Mortal Wkly Rep 2013;62:645-648.


31. Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med 2012;156:263-270.



32. Centers for Disease Control and Prevention (CDC). Surveillance for Viral Hepatitis ŌĆō United States, 2011. CDC web site, <https://www.cdc.gov/hepatitis/Statistics/2011Surveillance/Commentary.htm>. Accessed 1 Jun 2021.
33. Havens PL, Anderson JR. Updated CDC recommendations for universal hepatitis C virus screening among adults and pregnant women: implications for clinical practice. JAMA 2020;323:2258-2259.


34. Eckman MH, Ward JW, Sherman KE. Cost effectiveness of universal screening for hepatitis C virus infection in the era of direct-acting, pangenotypic treatment regimens. Clin Gastroenterol Hepatol 2019;17:930-939.e9.


35. Higuchi M, Tanaka E, Kiyosawa K. Epidemiology and clinical aspects on hepatitis C. Jpn J Infect Dis 2002;55:69-77.

36. Nakamura J, Terajima K, Aoyagi Y, Akazawa K. Cost-effectiveness of the national screening program for hepatitis C virus in the general population and the high-risk groups. Tohoku J Exp Med 2008;215:33-42.


37. Nagai K, Ide K, Kawasaki Y, Tanaka-Mizuno S, Seto K, Iwane S, et al. Estimating the cost-effectiveness of screening for hepatitis C virus infection in Japan. Hepatol Res 2020;50:542-556.


38. Sroczynski G, Esteban E, Conrads-Frank A, Schwarzer R, M├╝hlberger N, Wright D, et al. Long-term effectiveness and cost-effectiveness of screening for hepatitis C virus infection. Eur J Public Health 2009;19:245-253.


39. Harris RJ, Harris HE, Mandal S, Ramsay M, Vickerman P, Hickman M, et al. Monitoring the hepatitis C epidemic in England and evaluating intervention scale-up using routinely collected data. J Viral Hepat 2019;26:541-551.



40. Williams J, Miners A, Harris R, Mandal S, Simmons R, Ireland G, et al. Cost-effectiveness of one-time birth cohort screening for hepatitis C as part of the national health service health check program in England. Value Health 2019;22:1248-1256.


41. Deuffic-Burban S, Huneau A, Verleene A, Brouard C, Pillonel J, Le Strat Y, et al. Assessing the cost-effectiveness of hepatitis C screening strategies in France. J Hepatol 2018;69:785-792.


42. Wong WWL, Tu HA, Feld JJ, Wong T, Krahn M. Cost-effectiveness of screening for hepatitis C in Canada. CMAJ 2015;187:E110-E121.



43. World Health Organization (WHO). Draft global health sector strategy on viral hepatitis, 2016ŌĆÉ2021. Geneva: WHO; 2016.
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement
Supplement Print
Print





