INTRODUCTION
Percutaneous local treatment with radiofrequency ablation (RFA) is the current standard of care for patients with early-stage hepatocellular carcinoma (HCC) unsuitable for surgical resection. Recently, overall survival rates for HCC less than 3 cm in diameter treated using RFA or surgical resection have been reported as comparable [
1]. RFA is indicated for patients with up to three HCCs Ōēż3 cm in diameter and Child-Pugh class A or B liver function [
2,
3]. However, the curability of RFA depends on its location. For example, a perivascular location has a high risk of incomplete ablation because of the heat sink effect in which cooling is provided by blood flow through vessels adjacent to the tumor [
4,
5]. As a result, the local tumor progression rate after RFA is approximately 30% at 3 years [
2]. This level is insufficient for curative treatment. A more curative ablation procedure is thus required.
Microwave ablation (MWA) is another percutaneous thermal ablation therapy. In comparison with RFA, MWA offers the advantages of faster heating and less susceptibility to heat sink effects because higher temperatures are generated [
6]. However, conventional MWA shows some major disadvantages. First, the ablation zone shows a teardrop shape. For that reason, MWA carries a high risk of thermal damage to the subcutaneous tissues and skin in ablation of the subcapsular tumor, especially with the development of systems with greater power [
6]. Second, the ablation size is unpredictable with changes in surrounding tissues [
6]. To overcome these disadvantages, EmprintŌäó (Covidien, Boulder, CO, USA) was developed as a new, next-generation MWA system with thermosphere technology. Microwave thermosphere ablation (MTA) is able to create predictable spherical zones of ablation by incorporating thermal control, field control, and wavelength control technologies into the system [
7]. Vogl et al. [
8] demonstrated that larger minimal ablative margins can be obtained by MTA in comparison to two routinely used conventional MWA systems. MTA is thus expected to safely improve local tumor control compared to RFA. This system was approved for use in the United States in April 2014, and for use in Japan in July 2017. The aim of the present study was to elucidate whether this new, next-generation MTA could safely improve outcome compared to RFA.
DISCUSSION
This retrospective cohort study evaluated whether a next-generation MTA for small HCC could safely improve local tumor control compared to RFA. In our clinical practice, as MTA was introduced in December 2017, and RFA was fully replaced by MTA from September 2018, the RFA group represents historical controls. As a result, the fact that local tumor progression rate was significantly reduced after introducing MTA for HCC in our clinical practice is noteworthy.
Regarding the comparison of local tumor control between RFA and conventional MWA, Facciorusso et al. [
12] demonstrated in a meta-analysis that the overall local tumor progression rate was similar between conventional MWA and RFA groups (odds ratio, 1.01; 95% CI, 0.53ŌĆō1.87;
P=0.98). Poulou et al. [
13] indicated in their review article that the results concerning local disease control rates with conventional MWA and RFA remain controversial. Clinical data about local tumor control are lacking for MTA. Takahashi et al. [
14] reported that the short-term local tumor progression rate compared favorably with that reported for RFA and other conventional MWA technologies in the literature. However, Suwa et al. [
15] could not identify a significant difference in local tumor progression rate between RFA and MTA groups in a short-term observation of a limited number of patients (log-rank test,
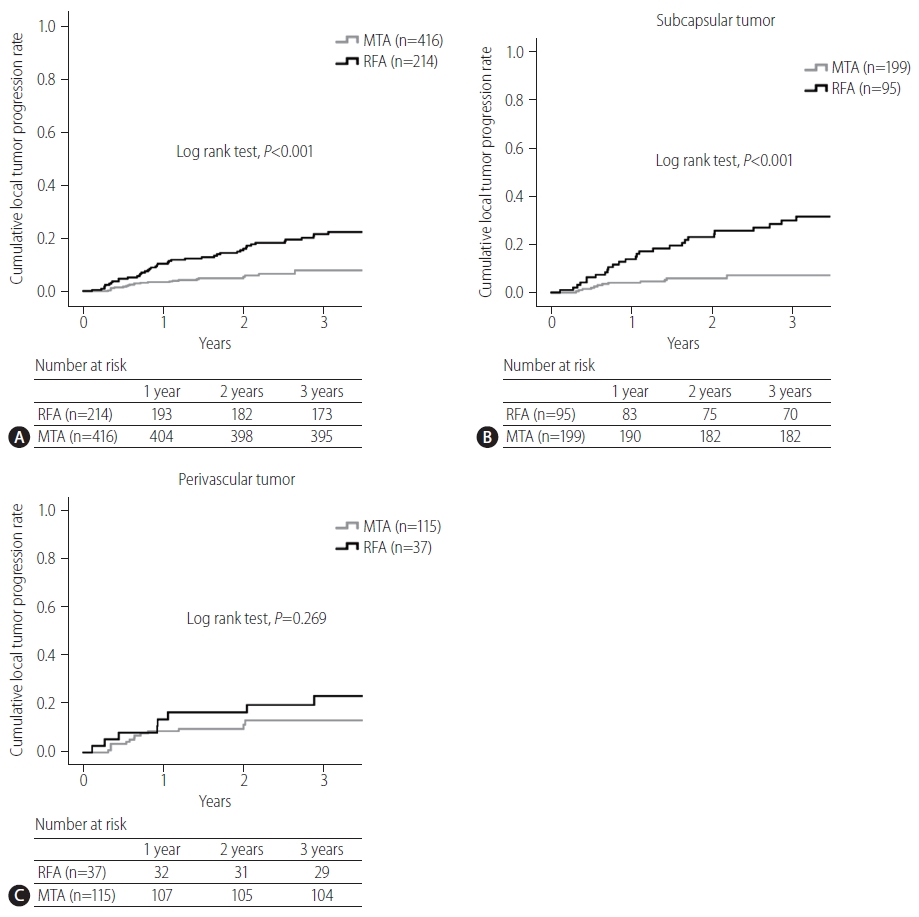
P=0.804, 1-year, 5.2 vs. 6.9%). The present study was able to show that the local tumor progression rate was significantly lower in the MTA group than in the RFA group (log-rank test,
P<0.001, 3-year, 22% vs. 8%).
Tumor location is one potential factor influencing the technical success and complications of RFA. Ablation for a perivascular tumor carries higher risks of injuring GlissonŌĆÖs sheath and achieving incomplete ablation because of heat sink effects, while ablation in a subcapsular location increases the difficulty of electrode placement and carries a higher risk of complications such as seeding or injuring adjacent organs. Lai et al. [
16] indicated that a subcapsular location represents a possible risk factor for local tumor progression after RFA on meta-analysis, and whether a perivascular location influences the incidence of local tumor progression remains controversial. In the present study, the local tumor progression rate of MTA for subcapsular tumor was significantly lower than that of RFA. This result suggests that MTA should be applied for subcapsular HCCs in place of RFA. Regarding a perivascular location, An et al. [
17] reported that MWA tended to exhibit better local tumor control than RFA for small perivascular HCCs, although the difference was not significant (
P=0.116). The present study likewise failed to show any significant difference in local progression rate between RFA and MTA for perivascular HCC. The reason may be attributable to the fact that MTA was still slightly affected by heat sink effects. Further study is needed to clarify whether MTA can reduce the risk of local tumor progression after ablation for perivascular HCCs. Our better local control of tumor with MTA compared to RFA, particularly for subcapsular tumors, may have been attributable to reliable coagulation and a larger ablated margin rather than to reduced susceptibility to heat sink effects from peritumor vessels, artificial pleural effusion or ascites.
Regarding the comparison of safety between RFA and conventional MWA, a meta-analysis by Facciorusso et al. [
12] demonstrated that major complications tended to be more frequent in MWA patients, although not significantly (odds ratio, 1.63; 95% CI, 0.88ŌĆō3.03;
P=0.12). Comparing safety between RFA and MTA, Suwa et al. [
15] reported no significant differences in incidence rates of complications between groups (MTA vs. RFA, 13.6% vs. 14.5%). However, in the present study, the total incidence of complications was significantly lower with MTA than with RFA, particularly for bile duct injury. This result was attributed to the high predictability of the ablation zone. Planning with accurate prediction of the ablation zone would seem likely to achieve safer ablation.
Among factors potentially associated with local tumor progression in RFA, tumor size is the only significant predictor [
2]. In the present study, not only tumor size but also treatment procedure were factors independently contributing to local tumor progression (MTA,
P<0.001; HR, 0.277; 95% CI, 0.155ŌĆō0.494). Subcapsular and exophytic tumors were significant factors on univariable analysis, but not on multivariable analysis. These location factors would probably be confounders for tumor size.
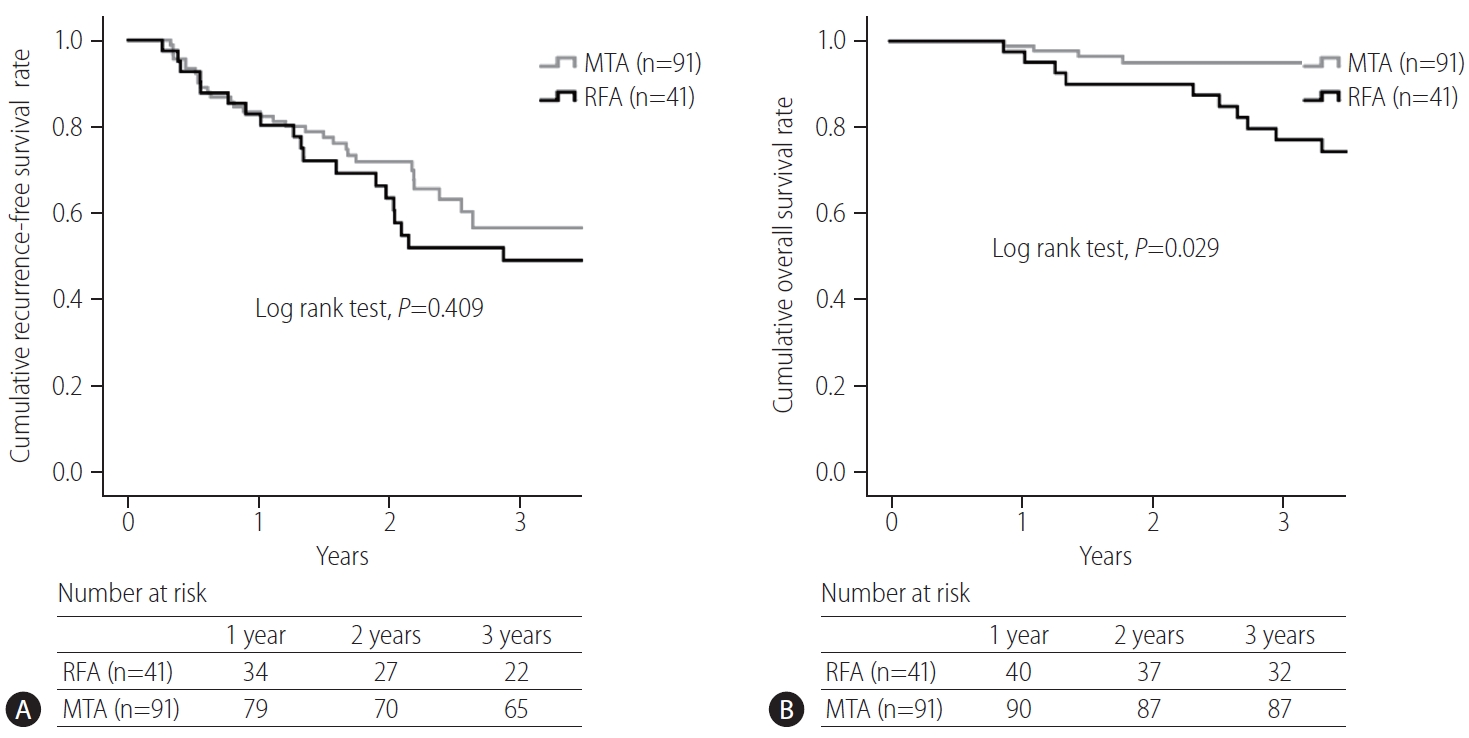
In the present study, no significant difference in recurrence-free survival was seen between RFA and MTA groups (RFA vs. MTA, 3-year, 49% vs. 57%). Kim et al. [
18] demonstrated that the cumulative recurrence rate in patients with early-stage HCC who received curative treatment such as RFA or resection was around 50% at 3 years. As most recurrent HCC after curative treatment is ectopic, MTA cannot be said to have the ability to decrease multicentric or metastatic recurrences.
Regarding the comparison of overall survival between RFA and conventional MWA, Facciorusso et al. [
12] demonstrated in their meta-analysis that the overall survival rate after RFA tended to be higher, without showing a significant difference. Poulou et al. [
13] also indicated in their review article that survival rates were generally comparable between MWA and RFA groups, within the range of 68ŌĆō100% after the first year and 24ŌĆō78% after the fourth year. In the present study, the overall survival rate after MTA was significantly higher than that after RFA. Furthermore, treatment procedure was an independent factor contributing overall survival. Sala et al. [
19] reported that initial complete response without local tumor progression to percutaneous ablation is associated with an improved survival in both Child-Pugh class A and B patients with nonsurgical HCC. As the local tumor progression rate after MTA was significantly lower than that after RFA, MTA might improve the prognosis over RFA. In the present study, however, as the proportion of liver cirrhosis and AFP level were lower in the MTA group than in the RFA group, better prognosis after MTA may be expected.
The present study showed several limitations. First, as the present study was a single-center, retrospective study, the potential for selection bias is unavoidable. A larger-scale randomized trial is needed to validate our results. Second, patient recruitment periods differed between the MTA and RFA groups. In particular, significant differences in proportions of perivascular tumor, liver cirrhosis, and etiology were seen between groups. Comparison after matching for important characteristics is therefore required. Third, local tumor control and safety of MTA in large HCCs >3 cm in diameter could not be evaluated in the present study. Izzo et al. [
20] suggested in a review article that MWA should be considered the technique of choice in selected patients, when the tumor is Ōēź3 cm in diameter. Future studies should clarify whether MTA should be applied to large HCC Ōēź3 cm in diameter.
In conclusion, this next-generation MTA for small HCC provided safer and more curative treatment than RFA within a shorter ablation time. MTA can also reduce the burden on not only patients, but also medical staff through this shorter ablation time. MTA has potential to replace RFA for small HCC.






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



