| Clin Mol Hepatol > Volume 20(4); 2014 > Article |
ABSTRACT
Bi-phenotypic neoplasm refers to tumors derived from a common cancer stem cell with unique capability to differentiate histologically into two distinct tumor types. Bi-phenotypic hepatocellular carcinoma-cholangiocarcinoma (HCC-CC), although a rare tumor, is important for clinicians to recognize, since treatment options targeting both elements of the tumor are crucial. Imaging findings of bi-phenotypic HCC-CC are not specific and include features of both HCC and CC. A combination of imaging and immuno-histochemical analysis is usually needed to make the diagnosis.
Composite tumors are typically comprised of two tumors each of different origin, pathology and phenotype in close proximity-which results in actual histologic intermingling of tumor cells. The presence of two different cell types can lead to perplexing imaging findings, which at times makes the diagnosis challenging and may necessitate a biopsy for confirmation.1 In contrast, collision tumors refer to two distinct neoplasms, usually of different biological behavior and histology, which coexist within a single organ. In contrast to composite tumors, collision tumors remain histologically distinct.2 Although rare, it is important to clinically recognize these tumors since if biopsy shows only the benign component, management of the tumor can result in insufficient patient care with possible adverse consequences.2
There are many theories explaining the pathogenesis of collision and composite tumors. One logical explanation is that of coincidentally occurring primary neoplasms integrating due to proximity and contiguity.2 Another hypothesis suggests that two different tumors originate in a common location, due to an altered cellular microenvironment activated by common carcinogenic stimuli.3 This is illustrated with one of the classic bi-phenotypic tumors, combining both hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC) seen on a common background of chronic parenchymal disease caused by the hepatitis virus.4 A third hypothesis suggests that microenvironment changes created by the first tumor eventually leads to the development of the second tumor.4
This short review illustrates the imaging appearances and pathologic correlation of bi-phenotypic composite liver tumors.
Bi-phenotypic neoplasm refers to tumors derived from a common cancer stem cell with unique capability to differentiate histologically into two distinct tumor types. Tumor cells express both hepatocellular and biliary markers by immunohistochemistry and may also express progenitor cell and stem cell markers.5
One of the more commonly described composite tumors in the liver is the co-existence of HCC with CC. Combined HCC-CC is a rare primary liver tumor showing dual hepatocellular and biliary epithelial differentiation.5 This type of lesion constitutes less than 1% of all HCCs.6 Interestingly, not all these lesions arise on a background of cirrhosis. Electron microscopic studies have confirmed the presence of dual differentiation with venous and parenchymal invasion, into adjacent liver parenchyma and microsatellite formation, as seen in HCC.7 Studies have suggested that combined HCC-CC is genetically more similar to CC than HCC with common carcinogenesis pathways altered in HCC-CC and CC.8
HCC-CC is a rare lesion; thus not much is known about imaging features, management and prognosis. Several studies have shown worse outcomes overall in HCC-CC when compared with either HCC or CC.8 Serum tumor markers, such as alpha-fetoprotein (╬▒FP) and carbohydrate antigen 19-9 (CA-19-9) may be helpful when imaging findings are equivocal. However, knowledge of radiological imaging features of these lesions is essential for correct management and clinical care.
Imaging is unusual in that the HCC component may not show the classic appearance of brisk arterial phase enhancement with subsequent washout on the portal venous and delayed phases.5 In Figure 1, a tumor shows no significant arterial enhancement typical of HCC because of a predominant CC component.9 In this case, progressive enhancement of central portion of the tumor, which is a typical finding of intrahepatic CC, can be noted.
The CC component may also not show associated biliary dilatation or delayed enhancement; although, mild enhancement during the portal venous phase with persistent enhancement on the delayed phase is seen sometimes due to predominant fibrous component as seen in Figure 2.10
Classification systems of combined HCC-CC tumor CT findings have been described by Aoki et al. and Sanada et al. Aokie et al. describes combined HCC-CC divided into either type A and type B categories. Type A is classified as a tumor that has peripheral enhancement in early phase with hyper-enhancement in the central portion of the tumor and peripheral washout in the delayed phase. Type B is classified as a tumor that has enhancement on the arterial phase and then washout on the delayed phase, following the HCC tumor pattern of enhancement.11
Sanada et al has described 3 different types of enhancement patterns. Type 1 is characterized with early enhancement followed by washout in the portal venous delayed phase, characteristic of HCC. Type 2 is characterized with peripheral enhancement in every phase. Type 3 is characterized as containing the enhancement patterns of both HCC and CC, with the HCC component showing early enhancement with delayed washout and the CC component showing delayed enhancement.12
Similar to computed tomography, magnetic resonance imaging of bi-phenotypic HCC-CC shows mixed characteristics of HCC and CC as shown by Figure 3. These lesions generally show irregular peripheral rim enhancement on arterial with progressive filling in or progressive enhancement of a nodular component.13
Maximin et al. describes combined HCC-CC as hypointense on T1-weighted images while T2-weighted images show an increased signal intensity which may contain a hypointense focus, representing the CC component.14
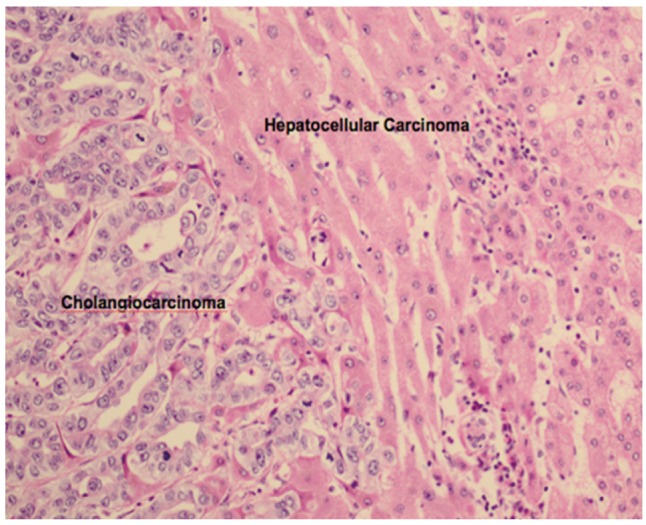
However, preoperative diagnosis of bi-phenotypic tumors with HCC and CC solely based on imaging may not be easy. Assessment of HCC and CC risk factors and tumor markers (╬▒FP and CA-19-9) can increase overall accuracy. In the absence of typical imaging features, biopsy is indicated for confirmation.5 On biopsy, immuno-histochemical analysis will show both hepatocellular and bile duct epithelium, as shown in Figure 4. This demonstrates that both the HCC and CC are derived from the same cancer stem cell with the capacity to differentiate into both liver and bile duct epithelium. The immunohistochemistry shows presence of both carcinoembryonicantien expressed by CC and Hep par 1 and ╬▒FP expressed by HCC, thus confirming the biophenotypic tumor.15
Fibrolamellar variant of HCC (FLHCC) occurring with CC is rare but has been reported in the literature.16 On imaging, the neoplasm demonstrates typical features of FLHCC; however on pathology, CC is admixed in with the FLHCC in both the primary tumor and metastatic lymph nodes. Immuno-histochemical analysis reveals that both the FLHCC and CC are derived from the same cancer stem cell with the capacity to differentiate into either liver or bile duct epithelium containing both cellular components, thereby exhibiting bi-phenotypic antigen expression. The coexistence of FLHCC and CC appears to be associated with a more aggressive clinical behavior of the tumor.
Studies have shown that bi-phenotypic HCC-CC has a worse prognosis as compared to HCC or CC. One study showed the median survival of patients with combined tumor to be 32 months while with HCC alone was 32 months and CC alone was 46 months.17 Treatment varies depending on clinical factors but can include surgical resection, interventional radiological procedures including transarterial chemoembolization and radiofrequency ablation as well as chemotherapy. A last resort treatment in certain cases may be liver transplantation.18 Despite treatment, tumor recurrence can occur within 6-9 months.5 Staging system of bi-phenotypic HCC-CC in American Joint Committee on Cancer cancer staging manual 7thediction complies with that of intrahepatic CC.
Acknowledgement
We thank Dr.Kumar Sandrasegaran, Prof of Radiology, Indiana University School of Medicine for cases.
REFERENCES
1. Thorin-Savoure A, Tissier-Rible F, Guignat L, Pellerin A, Bertagna X, Bertherat J, et al. Collision/composite tumors of the adrenal gland: a pitfall of scintigraphy imaging and hormone assays in the detection of adrenal metastasis. J Clin Endocrinol Metab 2005;90:4924-4929. 15914530.


2. Schwartz LH, Macari M, Huvos AG, Panicek DM. Collision tumors of the adrenal gland: demonstration and characterization at MR imaging. Radiology 1996;201:757-760. 8939227.


3. Katabathina VS, Flaherty E, Kaza R, Ojili V, Chintapalli KN, Prasad SR. Adrenal collision tumors and their mimics: multimodality imaging findings. Cancer Imaging 2013;13:602-610. 24434021.



4. Zhou YM, Zhang XF, Wu LP, Sui CJ, Yang JM. Risk factors for combined hepatocellular-cholangiocarcinoma: a hospital-based case-control study. World J Gastroenterol 2014;20:12615-12620. 25253966.



5. Shetty AS, Fowler KJ, Brunt EM, Agarwal S, Narra VR, Menias CO. Combined hepatocellular-cholangiocarcinoma: what the radiologist needs to know about biphenotypic liver carcinoma. Abdom Imaging 2014;39:310-322. 24407728.


6. Pua U, Low SC, Tan YM, Lim KH. Combined hepatocellular and cholangiocarcinoma with sarcomatoid transformation: radiologic-pathologic correlation of a case. Hepatol Int 2009;3:587-592. 19763713.



7. Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol 1998;13:34-40. 9737569.


8. Kim SH, Park YN, Lim JH, Choi GH, Choi JS, Kim KS. Characteristics of combined hepatocelluar-cholangiocarcinoma and comparison with intrahepatic cholangiocarcinoma. Eur J Surg Oncol 2014;40:976-981. 24909336.


9. Fowler KJ, Sheybani A, Parker RA 3rd, Doherty S, M Brunt E, Chapman WC, et al. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am J Roentgenol 2013;201:332-339. 23883213.


10. Fukukura Y, Taguchi J, Nakashima O, Wada Y, Kojiro M. Combined hepatocellular and cholangiocarcinoma: correlation between CT findings and clinicopathological features. J Comput Assist Tomogr 1997;21:52-58. 9022770.


11. Aoki K, Takayasu K, Kawano T, Muramatsu Y, Moriyama N, Wakao F, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology 1993;18:1090-1095. 7693572.


12. Sanada Y, Shiozaki S, Aoki H, Takakura N, Yoshida K, Yamaguchi Y. A clinical study of 11 cases of combined hepatocellular-cholangiocarcinoma Assessment of enhancement patterns on dynamics computed tomography before resection. Hepatol Res 2005;32:185-195. 15978872.


13. Hashimoto T, Nakamura H, Hori S, Tomoda K, Mitani T, Murakami T, et al. MR imaging of mixed hepatocellular and cholangiocellular carcinoma. Abdom Imaging 1994;19:430-432. 7950820.


14. Maximin S, Ganeshan DM, Shanbhogue AK, Dighe MK, Yeh MM, Kolokythas O, et al. Current update on combined hepatocellular carcinoma-cholangiocarcinoma. Eur J Radiol Open 2014;1:40-48.



15. Balaton AJ, Nehama-Sibony M, Gotheil C, Callard P, Baviera EE. Distinction between hepatocellular carcinoma, cholangiocarcinoma, and metastatic carcinoma based on immunohistochemical staining for carcinoembryonic antigen and for cytokeratin 19 on paraffin sections. J Pathol 1988;156:305-310. 2465399.


16. Tanaka K, Honna T, Kitano Y, Kuroda T, Tanaka K, Morikawa N, et al. Combined fibrolamellar carcinoma and cholangiocarcinoma exhibiting biphenotypic antigen expression: a case report. J Clin Pathol 2005;58:884-887. 16049296.



Figure┬Ā1
Multiphase computed tomography of HCC-CC. A) Arterial phase imaging, shows intense peripheral enhancement representing the hepatocellular component (red arrow). B) Portal venous phase shows progressive fill in tumor of tumor (red arrow). C) Delayed phase shows further fill in representing the cholangiocarcinoma component (red arrow).

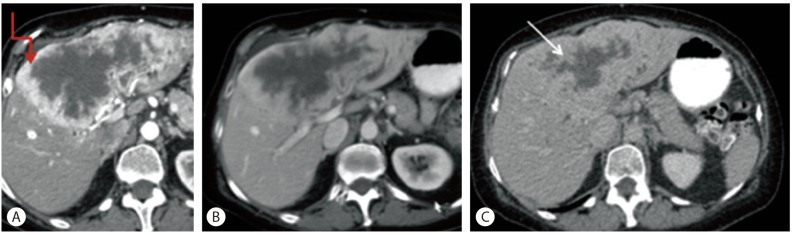
Figure┬Ā2
Computed tomography (A) Arterial phase shows a large heterogeneous, briskly peripherally enhancing liver mass. The briskly enhancing portion represents the hepatocellular component (red arrow). (B) Portal venous phase and (C) delayed phase show progressive fill in with enhancement getting more intense. The delayed enhancing portion represents the cholangiocarcinoma component (white arrow).
- TOOLS




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




