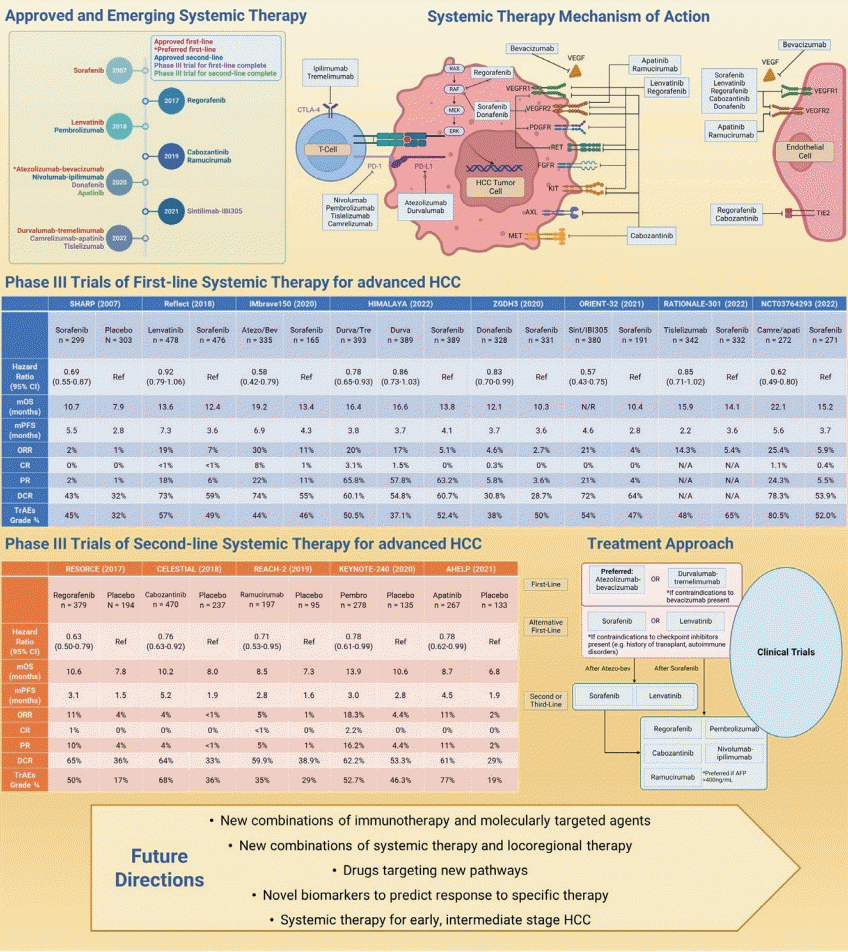
Systemic therapy in advanced hepatocellular carcinoma
-
Joseph C. Ahn1, Nguyen H. Tran2, Ju Dong Yang3,4,5

- Received February 10, 2023 Revised February 16, 2023 Accepted February 16, 2023
- FOOTNOTES
- FOOTNOTES
-
Authors’ contributions Ju Dong Yang devised the project and the main conceptual ideas for the snapshot; Joseph C. Ahn conducted the literature search and identified relevant studies to be included in the review; Ahn JC drafted the manuscript and the figure; Ju Dong Yang and Nguyen H. Tran revised the manuscript critically for important intellectual content; and all authors approved the final version to be published.
Conflicts of Interest Dr. Yang provides a consulting service for Exact Sciences and Gilead. Dr. Yang’s research is funded by National Institute of Health 1K08CA259534-01A1. Dr. Tran’s research is funded by National Institute of Health 1K23MD017217-01A1.
- Abbreviations
- Abbreviations
HCC hepatocellular carcinoma
PD-1 programmed cell death protein-1
PD-L1 programmed death-ligand 1
CTLA-4 cytotoxic T lymphocyte-associated protein 4
HR hazard ratio
OS overall survival
SBRT stereotactic body radiation therapy
- REFERENCES
- REFERENCES
REFERENCES
1. Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ 2020;371:m3544.
[Article] [PubMed]2. Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2022 Nov 11. doi: 10.1038/s41575-022-00704-9.
[Article] [PubMed]3. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al.; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390.
[Article] [PubMed]4. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-1173.
[Article] [PubMed]5. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al.; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66 Erratum in: Lancet 2017;389:36.
[Article] [PubMed]6. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63.
[Article] [PubMed] [PMC]7. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al.; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282-296.
[Article] [PubMed]8. Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallelcontrolled phase II-III trial. J Clin Oncol 2021;39:3002-3011.
[Article]9. Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol 2021;6:559-568.
[Article]10. El-Khoueiry AB, Melero I, Yau TC, Crocenzi TS, Kudo M, Hsu C, et al. Impact of antitumor activity on survival outcomes, and nonconventional benefit, with nivolumab (NIVO) in patients with advanced hepatocellular carcinoma (aHCC): Subanalyses of CheckMate-040. J Clin Oncol 2018;36(4 suppl):475.
[Article]11. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al.; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-952 Erratum in: Lancet Oncol 2018;19:e440.
[PubMed]12. Qin S, Kudo M, Meyer T, Finn RS, Vogel A, Bai Y, et al. Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol 2022;33(Suppl 7):S1402-S1403.13. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al.; IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894-1905.
[Article]14. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-873.
[Article] [PubMed]15. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol 2020;38:4317-4345.
[Article] [PubMed]16. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-693.
[Article] [PubMed] [PMC]17. Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al.; AASLD Panel of experts on trial design in HCC. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology 2021;73 Suppl 1:158-191.
[Article] [PubMed]18. Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol 2005;175:7746-7754.
[Article] [PubMed] [PMC]19. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol 2020;6:e204564 Erratum in: JAMA Oncol 2021;7:140.
[Article] [PubMed] [PMC]20. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence 2022;1:EVIDoa2100070.
[Article]21. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, openlabel, randomised, phase 3 trial. Lancet Oncol 2022;23:995-1008.
[Article] [PubMed]22. Finn MK R.S, Merle P, Meyer T, Qin S, Ikeda M, Xu R, et al. Pembrolizumab/lenvatinib combo misses survival end points in unresectable HCC. Ann Oncol 2022;33(Suppl 7):S1808-S869.23. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al.; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22:977-990 Erratum in: Lancet Oncol 2021;22:e347.
[Article] [PubMed]24. Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, et al. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol 2022;33(Suppl 7):S1401-S1402.25. Llovet JM, Vogel A, Madoff DC, Finn RS, Ogasawara S, Ren Z, et al. Randomized phase 3 LEAP-012 study: transarterial chemoembolization with or without lenvatinib plus pembrolizumab for intermediate-stage hepatocellular carcinoma not amenable to curative treatment. Cardiovasc Intervent Radiol 2022;45:405-412.
[Article] [PubMed] [PMC]26. Tai D, Loke K, Gogna A, Kaya NA, Tan SH, Hennedige T, et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:1025-1035.
[Article] [PubMed]27. Dawson LA, Winter K, Knox J, Zhu AX, Krishnan S, Guha C, et al. NRG/RTOG 1112: Randomized phase III study of sorafenib vs. stereotactic body radiation therapy (SBRT) followed by sorafenib in hepatocellular carcinoma (HCC). J Clin Oncol 2023;41(4 suppl):489.
[Article]