Preventive strategy for nonalcoholic fatty liver disease-related hepatocellular carcinoma
Article information
Abstract
The incidence of hepatocellular carcinoma (HCC) associated with nonalcoholic fatty liver disease (NAFLD) has been increasing worldwide, including Asia. Most patients with NAFLD-related HCC are at a much-advanced stage and older age at the time of diagnosis than those with virus-related HCC because they have not undergone HCC surveillance. This review provides an overview of the mechanism of hepatocarcinogenesis in NAFLD, preventive strategies for NAFLDrelated HCC, and strategies for the surveillance of patients with NAFLD.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is currently the leading cause of chronic liver disease in Korea, with an estimated prevalence of 20–30% among general population [1]. NAFLD is regarded as the hepatic manifestation of the metabolic syndrome and is also closely associated with diabetes, hyperlipidemia, obesity, and hypertension. Considering the trend of obesity in Korea [2], NAFLD may become more prevalent in the near future and may become an important etiology of chronic liver disease and liver cancer. As the prevalence of metabolic syndrome has notably increased [3], the prevalence of NAFLD has doubled in the last two decades to 30%. Although simple steatosis is often regarded as a non-progressive condition, 20–30% of patients with nonalcoholic fatty liver progress to chronic liver disease (nonalcoholic steatohepatitis [NASH]), which is characterized by hepatocyte injury, lobular inflammation, and fibrosis, and can result in liver cirrhosis (LC) (F4) in 20% of NASH patients with advanced fibrosis (F3) over 2 years [4,5]. NAFLD and NAFLD-related hepatocellular carcinoma (HCC) have received relatively little attention because cardiovascular events are the most common cause of death among patients with NAFLD. However, with the increase in the prevalence of metabolic syndrome and the decrease in the population with chronic hepatitis B or C worldwide, NAFLD, especially NASH, has increasingly become an important etiology of HCC [6].
Hepatocarcinogenesis in patients with NAFLD and NASH is complex and not fully understood. Although the progression to cirrhosis occurs before the development of HCC in the majority of chronic liver diseases, this is not always the case with NAFLD-related HCC, because HCC may develop even if cirrhosis is not definitively present [7]. The rate of NASH-associated hepatocarcinogenesis is approximately 1.5–2.6% per year [6].
PATHOGENESIS: PROPOSED MECHANISMS
Obesity and diabetes, which are two important risk factors for NAFLD, increase the risk of HCC [8]. The pathogenesis of HCC in patients with NAFLD (Fig. 1) is also independent of the presence of liver cirrhosis. Among patients with NAFLD, HCC may develop even in the absence of advanced hepatic fibrosis and histological inflammation.
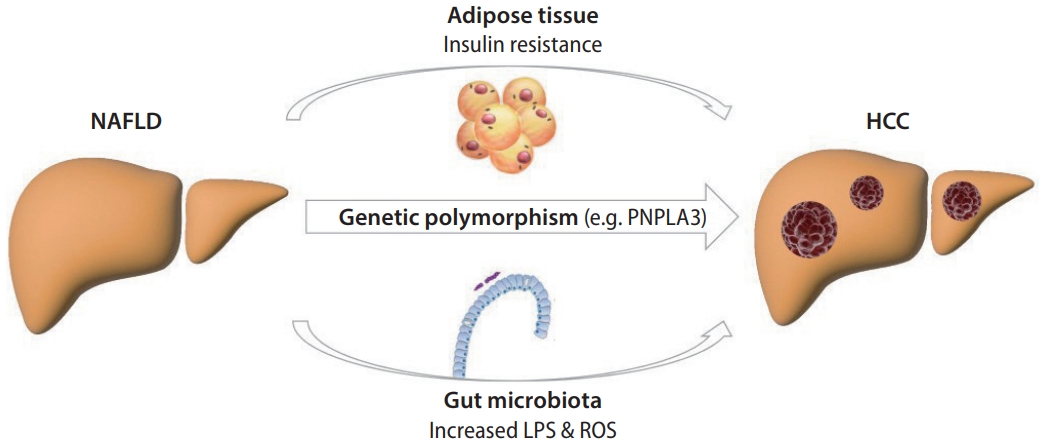
Pathogenesis of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. NAFLD, nonalcoholic fatty liver disease; HCC, hepatocellular carcinoma; LPS, lipopolysaccharide; ROS, reactive oxygen species.
The association between obesity and HCC among patients with NAFLD has also been proven for HCC in a previous study in the United States, which included more than 900,000 persons. The individuals were stratified according to their body mass index (BMI). The relative risk of mortality of HCC was 4.52 and 1.90 in patients with obesity grade II and I, respectively [9]. A study from Korea with 700,000 participants also confirmed an increased risk (relative risk, 1.56) of HCC in patients with BMI >30 kg/m2 [10]. A persistent, low-grade inflammatory response due to obesity and an abundance of adipose tisssue are thought to be key factors in hepatocarcinogenesis [11]. Increased levels of leptin, a proinflammatory, proangiogenic, and profibrogenic cytokine that promotes growth by activating the Janus kinase pathway [12], are a result of obesity. Adiponectin, an anti-inflammatory cytokine, is decreased in obesity [13-15]. Lipotoxicity, which results from lipid accumulation in the liver, causes the development of reactive oxygen species, endothelial reticulum stress, and saturated and monounsaturated free fatty acids. Free fatty acids can disrupt cellular signaling pathways causing changes in gene transcription [16]. By activating numerous carcinogenic pathways, insulin and insulin-like growth factor may aid in the development of primary liver cancer [17].
Insulin resistance and hyperinsulinemia also increase toxic metabolites in hepatocytes [18]. Hyperglycemia modifies the cell vasculature, leading to defects in endothelial cells. Endothelial damage leads to impaired fibrinolytic capacity, increased growth factor production, increased levels of adhesion molecules and inflammatory cytokines, increased reactive oxygen species, and enhanced cellular permeability [19]. Insulin resistance also leads to hyperinsulinemia, which triggers the production of free fatty acids and reactive carbonyl compounds in adipose tissue [20]. Advanced glycation end-products in hepatocytes aggravate oxidative stress and DNA damage, which are the probable consequences of hepatocarcinogenesis [21].
The alteration of the gut microbiota in patients with NAFLD also leads to hepatocarcinogenesis [22]. The level of lipopolysaccharide, which is the main component of the outer membrane of gram-negative bacteria, increases with obesity. Interestingly, further evidence of the role of lipopolysaccharide in hepatocarcinogenesis is derived from the finding that gut sterilization and lipopolysaccharide removal reduce HCC development in the chronically damaged liver [23,24].
The development of HCC in NAFLD may also be influenced by genetic variation. The minor allele of PNPLA3 rs738409 c.444C>G (encoding the I148M variant) has been linked to hepatocarcinogenesis. This polymorphism provides an elevated risk in the absence of potentially confounding covariates such as age, sex, coexisting diabetes, obesity, and cirrhosis [25,26].
PREVENTION OF NAFLD-RELATED HCC
Several risk factors associated with hepatocarcinogenesis in the NAFLD population may be reduced by lifestyle interventions or chemoprevention; however, the benefits of these approaches are likely to extend beyond risk factor modification. Changes in lifestyle and management of metabolic risk factors may help prevent HCC. Further epidemiological studies are required to tailor screening strategies, particularly in noncirrhotic populations with NAFLD.
Weight reduction
The primary treatment for the majority of patients with NAFLD is weight reduction. However, weight loss has not been directly proven to reduce the incidence of NAFLD-related HCC. Previous clinical studies have demonstrated that weight loss positively influences NAFLD activity, with some data indicating the possibility of hepatic fibrosis regression. Weight reduction for all patients with NAFLD is recommended, especially those who are overweight (BMI >25 kg/m2) or obese (BMI >30 kg/m2), because weight loss at a rate of 0.5–1.0 kg/week can lead to improvement in biochemical tests, serum insulin levels, and liver histology [27-30]. Weight reduction of 5–7% leads to lower intrahepatic fat content in NAFLD patients, and weight loss of 7–10% is necessary to ameliorate hepatic inflammation and fibrosis [1].
The following are the behavioral adjustments for obese patients: (1) consuming a low-calorie, low-fat diet; (2) regular participation in moderate physical activity; and (3) regular checking of body weight and abdominal circumference.
Physical activity
HCC risk reduction has recently been found in the European Prospective Investigation into Cancer and Nutrition cohort study among subjects who engaged in at least 2 hours of intense exercise each week with a hazard ratio (HR) of 0.5, independent of body weight and other common risk factors for HCC [31]. A meta-analysis of 14 prospective studies also indicated a considerably decreased risk of liver cancer in those with high physical activity compared to those with low physical activity (HR, 0.75; 95% confidence interval [CI], 0.63– 0.89) [32].
Dietary modification
Among dietary patterns, higher adherence to the Mediterranean diet substantially lowered the risk of HCC (odds ratio, 0.51 [33]; HRs, 0.62 [34] and 0.68 [35]). The Mediterranean diet is also recommended by European and Korean guidelines for NAFLD [1,36].
Coffee is a dietary component that has shown potential for the treatment of both NAFLD and HCC. People who drank coffee at least twice a day had a considerably decreased incidence of HCC than non-drinkers (HR, 0.40; 95% CI, 0.20–0.79) [37]. A meta-analysis of six Japanese cohort studies corroborated this finding, with a pooled relative risk estimate of 0.50 (95% CI, 0.38–0.66) for frequent coffee drinking vs. no coffee consumption [38].
It has also been proposed that dietary antioxidants (vitamins C and E, as well as selenium) may help reduce hepatocarcinogenesis [39]. This may particularly important given that patients with NASH have been shown to have vitamin E and D insufficiency [40], and that vitamin D deficiency may play a role in hepatocarcinogenesis [41].
PHARMACOLOGIC PREVENTION
Several pharmacological treatments have been reported to modify risk variables and carcinogenic pathways in NAFLD-associated HCC, indicating their potential use in preventive pharmacological strategies. In this section, the pharmacological treatments that have been shown to prevent HCC are reviewed. There are few studies that have verified the chemopreventive effect only on NAFLD patients. Therefore, clinicians should be careful in interpreting the routine use of drugs such as metformin and statin as prophylactic therapy in patients with NAFLD.
Aspirin
In large prospective population-based observational studies, aspirin and other antiplatelet medications have been shown to lower the risk of HCC [42-44]. Most studies have found that aspirin might exert a hepatitis B virus (HBV)-specific chemopreventive effect on HCC development. However, recent studies have also suggested that aspirin might have a preventive effect on NAFLD-related HCC.
A recent pooled analysis of two prospective United States cohort studies (the Nurses’ Health Study and the Health Professionals Follow-up Study) analyzed 133,371 participants. This study reported that regular, long-term aspirin use was associated with a reduction in HCC risk in a dose-dependent manner, which was apparent after ≥5 years of use. Interestingly, similar associations were not found with non-aspirin nonsteroidal anti-inflammatory drugs [43]. The analysis of this study was not limited to those with NAFLD, but considering that one dominant HCC risk factor in the Unites States is NAFLD, it can be accepted as a significant result. A prospective study of 361 patients with biopsy-proven NAFLD also reported that daily aspirin use was associated with a significantly lower risk of advanced fibrosis compared to non-regular aspirin use (adjusted HR, 0.63; 95% CI, 0.43–0.85) [45]. A recent systematic review and meta-analysis analyzing 19 observational studies also supported the preventive effect of aspirin on HCC development [46].
The ideal dose and duration of aspirin for preventing HCC incidence are still uncertain, and the chemopreventive impact of other nonsteroidal anti-inflammatory medications other than aspirin on HCC is unknown. Future studies are needed to determine the chemopreventive effects of aspirin in NAFLD and NASH patients.
Metformin
Metformin suppresses hepatic fat formation and glucose excretion by activating adenosine monophosphate-activated protein kinase; it also reduces tumor necrosis factor expression. In a subanalysis of a meta-analysis [47] analyzing 37 studies, a substantial decrease in HCC risk in diabetic patients was observed among metformin users in terms of HCC incidence (78%) and death (77%). Another meta-analysis of 10 studies that included 22,650 HCC cases among 334,307 diabetic individuals found that metformin treatment was associated with a 41% decrease in HCC incidence [48]. Metformin appears to have antitumoral effects via several pathways by decreasing the level of insulin-like growth factor-1, suppressing c-Jun N-terminal kinase/p38 mitogen-activated protein kinase, human epidermal growth factor receptor-2, and nuclear factor-κB pathways, activating AMP-activated protein kinase, inhibiting the mammalian target of rapamycin pathway, and decreasing the endogenous production of reactive oxygen species.
Statins
The protective impact of statins on HCC development is most likely due to their anti-inflammatory characteristics, which are mediated through Janus kinase inhibition [49]. Several clinical studies have found that statins are useful in lowering the risk of HCC [50-52]. A recent meta-analysis of 24 studies found that statin users had a 46% lower risk of HCC, indicating that statins might be used in chemoprophylaxis [53]. A subanalysis of another meta-analysis found that using lipophilic statins (atorvastatin, pitavastatin, lovastatin, fluvastatin, simvastatin) was linked with a considerably lower risk of HCC when compared to hydrophilic statins (rosuvastatin, pravastatin) (27% vs. 51%) [54]. Lipophilic statins have higher lipid solubility and membrane permeability, allowing them to have cholesterol-dependent effects on HCC development [55].
SURVEILLANCE STRATEGY FOR NAFLD
The annual incidence of HCC in individuals with NAFLD-related cirrhosis is greater than 1.5% [56,57]. If liver cirrhosis is clinically suspected among patients with NAFLD, HCC surveillance is recommended [58-60]. Since NAFLD-related LC patients may lose weight when they progress to LC, the etiology of cryptogenic LC should not be judged based on BMI alone. Non-invasive modalities to diagnose advanced fibrosis such as transient elastography might be a good tool to discriminate those high-risk population [1]. As shown in the previous systemic review [61], the incidence of HCC was quite low in subjects with early liver fibrosis (F0–2), 2.7% at 10 years and 23 per 100,000 person-years. However, patients with early liver fibrosis are more prone to develop HCC if they have other risk factors (obesity, metabolic syndrome, diabetes, etc.) and also HBV or hepatitis C virus infection in terms of metabolic-associated fatty liver disease. Therefore, a surveillance strategy for NAFLD patients should be individualized [58,62].
Although some evidence suggests that HCC can develop in livers without cirrhosis or steatohepatitis, surveillance should be carefully planned. Owing to the lack of robust data on the noncirrhotic population, it is difficult to develop evidence-based, cost-effective surveillance strategies for the NAFLD population. Clinical trials are needed to address the issue of surveillance in NAFLD, particularly in noncirrhotic persons [63].
Abdominal ultrasonography is the primary tool used for HCC surveillance. However, it might be difficult to accurately execute this procedure in overweight or obese patients [64,65]. Computed tomography or magnetic resonance imaging can be used instead.
CONCLUSION
Weight loss, dietary changes, and increased physical activity continue to be the cornerstones of HCC prevention in patients with NAFLD. The impact of lifestyle factors and chemopreventive agents may differ between NAFLD-associated hepatocarcinogenesis and hepatocarcinogenesis due to other etiologies, taking into account the heterogeneity of the NAFLD and NASH populations. A better understanding of the underlying pathophysiological mechanisms and disease phenotypes may enable focused preventive interventions for NAFLD-associated HCC in the future. New insights into the etiology, pathogenesis, and surveillance of HCC in patients with NAFLD may enable the development of therapeutic and preventive strategies.
Notes
Authors’ contribution
Study conceptualization: YC and JWP; Drafting of the manuscript: YC; Critical revision of the manuscript: BHK, YC, and JWP
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This work has supported by grants from the National Research Foundation of Korea grant funded by the Korea government (2021R1A2C4001401), the National Cancer Center, Korea (NCC-2210420-1), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HI21C0240).
Abbreviations
BMI
body mass index
CI
confidence interval
HCC
hepatocellular carcinoma
HR
hazard ratio
LC
liver cirrhosis
NAFLD
nonalcoholic fatty liver disease
NASH
nonalcoholic steatohepatitis
