Effect of moderate-to-severe hepatic steatosis on neutralising antibody response among BNT162b2 and CoronaVac recipients
Article information
Abstract
Background/Aims
Studies of hepatic steatosis (HS) effect on COVID-19 vaccine immunogenicity are lacking. We aimed to compare immunogenicity of BNT162b2 and CoronaVac among moderate/severe HS and control subjects.
Methods
Two hundred ninety-five subjects who received BNT162b2 or CoronaVac vaccines from five vaccination centers were categorized into moderate/severe HS (controlled attenuation parameter ≥268 dB/m on transient elastography) (n=74) or control (n=221) groups. Primary outcomes were seroconversion rates of neutralising antibody by live virus Microneutralization (vMN) assay (titer ≥10) at day21 (BNT162b2) or day28 (CoronaVac) and day56 (both). Secondary outcome was highest-tier titer response (top 25% of vMN titer; cutoff: 160 [BNT162b2] and 20 [CoronaVac]) at day 56.
Results
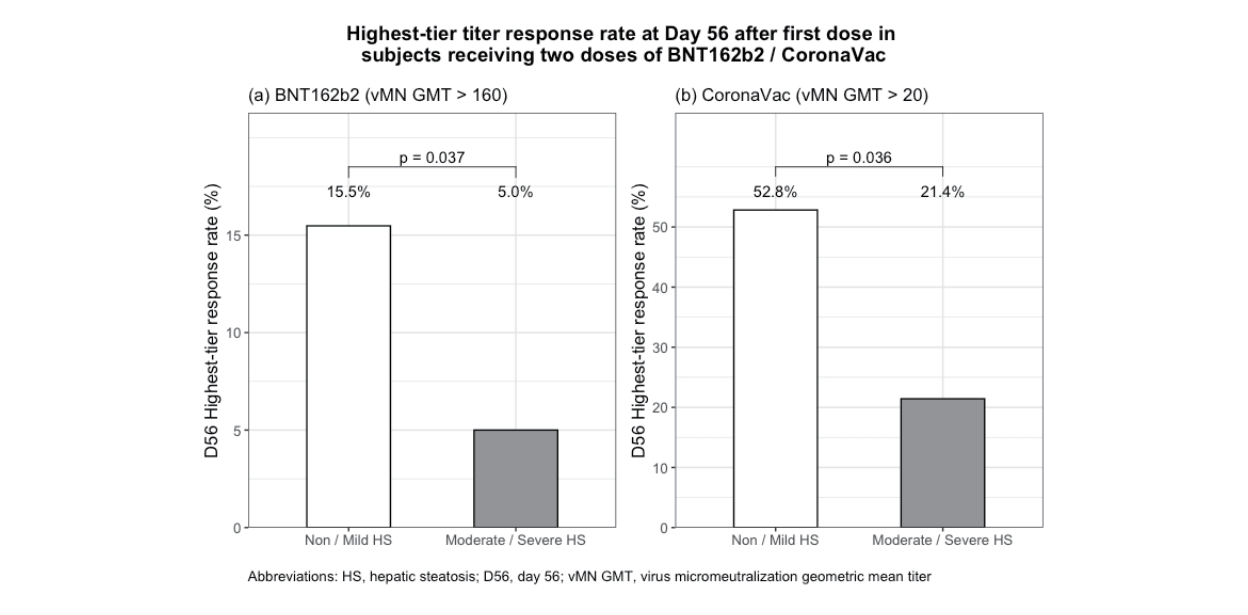
For BNT162b2 (n=228, 77.3%), there was no statistical differences in seroconversion rates (day21: 71.7% vs. 76.6%; day56: 100% vs. 100%) or vMN geometric mean titer (GMT) (day21: 13.2 vs. 13.3; day56: 91.9 vs. 101.4) among moderate/severe HS and control groups respectively. However, lower proportion of moderate/severe HS patients had highest-tier response (day56: 5.0% vs. 15.5%; P=0.037). For CoronaVac (n=67, 22.7%), there was no statistical differences in seroconversion rates (day21: 7.1% vs. 15.1%; day56: 64.3% vs. 83.0%) or vMN GMT (5.3 vs. 5.8,) at day28. However, moderate/severe HS patients had lower vMN GMT (9.1 vs. 14.8, P=0.021) at day 56 with lower proportion having highest-tier response (21.4% vs. 52.8%, P=0.036).
Conclusions
While there was no difference in seroconversion rate between moderate/severe HS and control groups after two doses of vaccine, a lower proportion of moderate/severe HS patients achieved highest-tier response for either BNT162b2 or CoronaVac.
Graphical Abstract
INTRODUCTION
The ongoing coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged into a global health burden. As of March 2022, COVID-19 has affected more than 400 million people and caused nearly 6 million deaths worldwide. Apart from measures such as social distancing, quarantine and isolation, vaccination is crucial in preventing infection, severe disease and death [1].
Chronic liver disease is associated with a higher infection risk and disease severity of COVID-19, especially those with liver cirrhosis [2]. With a global prevalence of 25% for non-alcoholic fatty liver disease (NAFLD) [3], concerns have been raised about COVID-19 vaccination response in this large population [4]. Since NAFLD is a multisystemic condition that presents with a wide spectrum of extrahepatic manifestations, such as obesity, diabetes mellitus (DM), cardiovascular diseases, the term metabolic-associated fatty liver disease (MAFLD) has recently been proposed to redefine NAFLD [5].
Neutralising antibody level is a surrogate marker of vaccine effectiveness [6] and is predictive of protection from symptomatic COVID-19 infection [7,8]. Recently, Wang et al. [9] reported that seroconversion rate of BBIBP-CorV (inactivated vaccine) was 95.5% after two doses of vaccine in 381 NAFLD patients. However, several questions remain unaddressed. First, it did not assess immunogenicity after single dose of vaccine and according to severity of hepatic steatosis (HS). Second, there were no control subjects without HS for comparison. Third, longer-term data (e.g., 6 months after vaccination) were not available. Fourth, studies evaluating the effect of mRNA vaccine and another commonly used inactivated vaccine (CoronaVac) in HS patients are currently lacking.
In Hong Kong, both mRNA and inactivated vaccines are available. Therefore, we aimed to conduct a longer-term prospective cohort study to compare immunogenicity of BNT162b2 and CoronaVac (after both first and second doses) and adverse events in patients with moderate-to-severe HS and control subjects.
MATERIALS AND METHODS
Study design
This is a prospective cohort study recruiting adult COVID-19 vaccine recipients (BNT162b2 and CoronaVac) from five local vaccination centers. Exclusion criteria included age <18 years, transplant patients, patients taking immunosuppressives/chemotherapy, inflammatory bowel disease, other medical diseases (cancer, hematological, rheumatological and autoimmune diseases), those with prior COVID-19 infection (identified from history taking or presence of antibodies to SARS-CoV-2 nucleocapsid protein which are not inducible by BNT162b2 and therefore is an indicator of past infection).
Severity of HS was defined by controlled attenuation parameter (CAP) ≥248 dB/m measured by transient elastography using Fibroscan® (Echosens, Paris, France): mild (CAP 248-267 dB/m), moderate (CAP 268-279 dB/m) and severe (CAP ≥280 dB/m) [10]. Since the metabolic and cardiovascular risks were significantly higher in significant fatty liver compared to mild fatty liver [11,12], we defined moderate/severe HS group (simplified as “HS” in subsequent sections) in our current study by CAP >268 dB/M. An alanine aminotransferase (ALT) level of 40 was used to identify possible non-alcoholic steatohepatitis (NASH).
Recruited subjects received either BNT162b2 or CoronaVac according to their preference. They received two doses of intramuscular BNT162b2 (0.3 mL) and CoronaVac (0.5 mL) 3 weeks and 4 weeks apart, respectively. Their blood samples were collected at four time-points: (i) before vaccination (baseline), (ii) 21 days (for BNT162b2) or 28 days (for CoronaVac) after first dose, (iii) 56 days after first dose (for both BNT162b2 and CoronaVac), and (iv) 180 days after first dose for BNT162b2 only (CoronaVac data was unavailable at this time point). Live virus Microneutralization (vMN) assay was performed in 96-well plate as described previously (Appendix 1) [13]. vMN positivity (seroconversion) was defined as titer ≥10 (31.25 IU/mL).
Subjects were requested to record any systemic and local events daily for 7 days after both first and second dose of vaccine. The severity of adverse events were graded as 1, 2, 3, and 4, according to toxicity grading scale by U.S. Department of Health and Human Services [14].
The study was approved by the Institutional Review Board of the University of Hong Kong (HKU) and Hong Kong West Cluster (HKWC) of Hospital Authority.
Outcome of interest
Primary outcomes of interest are seroconversion rates at three time points after first dose for BNT162b2 (day21, day56, and day180) and two time points for CoronaVac (day28 and day56).
Secondary outcomes of interest are (i) highest-tier titer response; (ii) overall and individual adverse events after both first and second doses. We defined the top 25% of vMN titer (i.e., above 75 percentile) as highest-tier titer response based on D56 data, while the remainders as having suboptimal humoral immune response. The 25% was an arbitrary cut-off as there is still no international consensus. A cut-off of 160 and 20 antibody tier was adopted for BNT162b2 and CoronaVac, respectively. Top 25% was selected as cut-off as vaccine effectiveness waned by time [15,16]. As Xu et al. [16] has reported a drop of neutralizing antibodies by one natural log unit from the peak at 6 months post infection, protection of vaccine among patients with higher antibody level is expected to last longer than those with lower antibody level. vMN titer at day 180 after first dose of BNT162b2 was measured to evaluate longer-term effect of BNT162b2.
Exposure of interest
Subjects with moderate or severe HS were grouped as “HS” and those with mild or no HS were regarded as control group. Covariates included age >70, sex, overweight/obesity, smoking, alcohol use, hypertension, DM, liver fibrosis (liver stiffness measurement >6.5 kPa) [17], concomitant renal impairment and past gastrointestinal surgery. Vaccine effectiveness is low until two doses of CoronaVac, and declines with increasing age among those aged >70 [18]. Overweight/obesity was defined as body mass index >23 according to World Health Organization and National Institutes of Health guidelines for Asians. Obesity may impair the ability to mount a protective immune response to influenza virus [19], and therefore COVID-19 vaccine immunogenicity. Diabetic patients have lower seropositivity rate of COVID-19 vaccination than healthy population [20]. Serum creatinine level is an useful indicator of metabolic syndrome in HS patients [21]. Renal impairment was defined as creatinine level >106 μmol/L for male and >97 μmol/L for female [22]. Since gut microbiota are involved in immune response to vaccination [23,24], prior gastrointestinal surgery that could possibly affect gut microbiota was included into analysis.
Sensitivity analysis was carried out by comparing moderate/severe HS subjects fulfilling criteria of MAFLD and control groups (subjects without HS or those with mild HS). The definition of MAFLD [5] was provided in Appendix 2.
Statistical analysis
All statistical analyses were conducted in R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) statistical software. Data is displayed as median (interquartile range [IQR]) for continuous variables, and as number of patients (percentage) for categorical variables. The Mann-Whitney U test was used for two continuous variables, and the chi-square test or Fisher exact test was used for categorical variables to assess the statistical significance between groups. Geometric mean titer (GMT) with 95% confidence interval (CI) was used to express the average vMN titer. A multivariable logistic regression model was used to estimate adjusted odds ratio (aOR) of highest-tier titer response with HS as well as all aforementioned covariates. For the purpose of statistical analysis, an MN titer of <10 was considered as 5. A two-sided P-value ≤0.05 was considered as statistically significant.
RESULTS
Demographics and baseline characteristics
A total of 295 subjects (moderate/severe HS: 74 [25.1%; 73 fulfilled the criteria of MAFLD] and control group: 221 [74.9%]) were enrolled. The demographics of subjects are shown in Table 1. Two hundred twenty-eight subjects received BNT162b2 (HS: 60 [26.3%] and control: 168 [73.7%]), while 67 subjects received CoronaVac (HS: 14 [20.9%] and control: 53 [79.1%]). Among BNT162b2 recipients, 27 (16.1%) of 168 subjects in control group had mild HS, and 13 (21.7%) of 60 subjects with moderate/severe HS had raised ALT. Among CoronaVac recipients, 11 (20.8%) of 53 subjects in control group had mild HS, and five (35.7%) of 14 subjects with moderate/severe HS had raised ALT.
The median age was similar between two groups, for either BNT162b2 (HS: 51.1 years vs. control: 50.9 years; P=0.242) or CoronaVac recipients (HS: 53.9 vs. control: 53.3 years; P=0.677). There were more males in the HS group than controls among both BNT162b2 recipients (55.0% vs. 29.8%; P<0.001) and CoronaVac recipients (50.0% vs. 24.5%; P=0.064). There was a higher proportion of HS patients being overweight/obese compared to controls (BNT162b2 group: 90% vs. 38.7%, P<0.001; CoronaVac group: 85.7% vs. 54.7%, P=0.034).
HS-associated comorbidities including hypertension, diabetes, alcohol intake were also included in Table 1. For BNT162b2 recipients, there were more HS patients who had DM compared to controls (18.3% vs. 3.6%, P<0.001). For CoronaVac recipients, there were more HS patients who had hypertension compared to controls (35.7% vs. 11.3%, P=0.028), and a higher proportion of HS patients had fibrosis than controls (21.4% vs. 1.9%, P=0.006). There were no significant difference in proportion of smokers (BNT162b2 group: 13.3% vs. 8.3%, P=0.260; CoronaVac group: 28.6% vs. 11.3%, P=0.107) and alcohol users (BNT162b2 group: 1.7% vs. 4.2%, P=0.366; CoronaVac group: 0% vs. 5.7%, P=0.362) among HS and control groups.
Comparison of vaccine immunogenicity between BNT162b2 and CoronaVac recipients
All subjects had vMN titer lower that of the detection limit. Table 2 shows that the seroconversion rate of BNT162b2 was higher than CoronaVac after both first dose (75.3% vs. 13.4%, P<0.001) and second dose (day56) (100% vs. 79.1%, P<0.001) for the whole cohort.
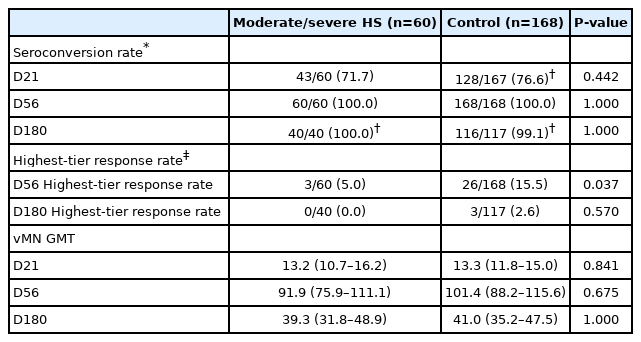
Among HS group, seroconversion rate of BNT162b2 was higher than CoronaVac after both first dose (71.7% vs. 7.1%, P<0.001) and second dose (100% vs. 64.3%, P<0.001). vMN GMT of BNT162b2 was higher than that of CoronaVac after both first dose (13.2; 95% CI, 10.7–16.2 vs. 5.3; 95% CI, 4.8–5.8; P<0.001) and second dose (91.9; 95% CI, 75.9–111.1 vs. 9.1; 95% CI, 6.8-12.1; P<0.001).
Among control group, seroconversion rate of BNT162b2 was higher than CoronaVac after both first dose (76.6% vs. 15.1%, P<0.001) and second dose (100% vs. 83%, P<0.001). vMN GMT of BNT162b2 was higher than that of CoronaVac after both first dose (13.3; 95% CI, 11.8–15.0 vs. 5.8; 95% CI, 5.3–6.6; P<0.001) and second dose (101.4; 95% CI, 88.2–115.6 vs. 14.8; 95% CI, 12.2–18.0; P<0.001).
Comparison of vaccine immunogenicity between moderate/severe HS and control groups among BNT162b2 recipients
Table 3 shows the humoral immune response among 228 BNT162b2 recipients. Fifty-nine (98.3%) of 60 subjects with moderate/severe HS had MAFLD. At day21, there was no significant difference in seroconversion rate among HS and control group (71.7% vs. 76.6%, P=0.442) or the vMN GMT (13.2 vs. 13.3, P=0.841). At day56, all vaccinees achieved seroconversion with a similar vMN GMT (91.9 vs. 101.4, P=0.675). However, there was a lower proportion of vaccinees having highest-tier titer response among the HS than control groups (5.0% vs. 15.5%, P=0.037). At day180, more than 99% remained seropositive with decreasing of vMN GMT from 91.9 to 39.3 in HS and from 101.4 to 41.0 in control group, and there was no longer significant difference in terms of highesttier titer response rate (0% vs. 2.6%, P=0.570). Supplementary Table 1 shows similar results by comparing MAFLD and control groups.
On univariate analysis, the OR of highest-tier titer response to BNT162b2 with HS was 0.29 (95% CI, 0.07–0.86). On multivariable analysis, HS was the only independent factor associated with this outcome (aOR, 0.24; 95% CI, 0.05–0.87) (Table 4).
Comparison of vaccine immunogenicity between moderate/severe HS and control groups among CoronaVac recipients
Table 5 shows the humoral immune response among 67 CoronaVac recipients. All moderate/HS subjects fulfilled criteria of MAFLD. At day28, there was no significant difference in seroconversion rate among HS and control group (7.1% vs. 15.1%, P=0.438) or the vMN GMT (5.3 vs. 5.8, P=0.420). At day56, all vaccinees achieved similar seroconversion rate among HS and control group (64.3% vs. 83.0%, P=0.125), but HS has lower vMN GMT than control group (9.1 [95% CI, 6.8– 12.1] vs. 14.8 [95% CI, 12.2–18.0], P=0.021). There was a lower proportion of vaccinees having highest-tier titer response among the HS than control groups (21.4% vs. 52.8%; P=0.036). Data of vMN GMT at day180 was not available for CoronaVac vaccinees.
On univariate analysis, OR of highest-tier titer response to CoronaVac with HS was 0.24 (95% CI, 0.05–0.88). Multivariable analysis did not reveal an association between HS and highest-tier antibody titer response.
Safety
After 1st dose vaccine
One hundred ninety (83.3%) BNT162b2 recipients and 36 (53.7%) CoronaVac recipients reported adverse events within 7 days of first dose of vaccine (Supplementary Table 2). All adverse events were mild (grade 1 and 2) and self-limiting. The most common local and systemic adverse events were injection site pain (78.1% for BNT162b2 and 35.8% for CoronaVac) and fatigue (30.7% BNT162b2 and 28.4% for CoronaVac).
Among BNT162b2 recipients, HS group showed higher rate of systemic reaction than control group (35 [58.3%] vs. 65 [38.7%], P=0.008). There was no significant difference in frequency of total adverse events and local reaction between HS and control groups (any adverse event: 50 [83.3%] vs. 140 [83.3%], P=1.0; local reaction: 47 [78.3%] vs. 133 [79.2%], P=0.892).
Among CoronaVac recipients, HS group showed lower rate of any adverse event (4 [28.6%] vs. 32 [60.4%], P=0.034) and local reactions (2 [14.3%] vs. 24 [45.3%], P=0.034) than control group. There was no significant difference in systemic adverse events between the two groups (systemic events: 3 [21.4%] and 22 [41.5%], P=0.167).
After 2nd dose vaccine
One hundred ninety-four (85.1%) BNT162b2 recipients and 30 (44.8%) CoronaVac recipients reported adverse events within 7 days of second dose of vaccine (Supplementary Table 2). All adverse events were mild (grade 1 and 2) and self-limiting. The most common local and systemic adverse events were injection site pain (76.8% for BNT162b2 and 29.9% for CoronaVac) and fatigue (42.1% BNT162b2 and 14.9% for CoronaVac).
Among BNT162b2 recipients, there was no significant difference in frequency of adverse events between HS and control groups (any adverse event: 51 [85%] vs. 143 [85.1%], P=0.982; local reaction: 44 [73.3%] vs. 135 [80.4%], P=0.256; systemic reaction: 36 [60%] vs. 92 [54.8%], P=0.483).
Among CoronaVac recipients, HS group showed lower rate of any adverse event (3 [21.4%] vs. 27 [50.9%], P=0.048) and local reactions (0 [0%] vs. 21 [39.6%], P=0.003) than control group. Lower rate of local adverse events in HS group was observed with injection site pain (0 [0%] vs. 20 [37.7%], P=0.007). There was no significant difference in systemic adverse events between the two groups (systemic events: 3 [21.4%] and 16 [30.2%], P=0.518).
DISCUSSION
This prospective cohort study demonstrates that BNT162b2 is more immunogenic than CoronaVac in terms of neutralising antibody response. While there was no difference in the seroconversion rate between moderate/severe HS and control groups for either BNT162b2 or CoronaVac, a lower proportion of the former group achieved a highest-tier humoral response after two doses of vaccines. Similar findings were observed when MAFLD patients with moderate/severe HS were compared with controls.
It has been suggested that patients with chronic liver disease display immune dysfunction in which predisposes them to infections, organ inflammatory damage and poor response to vaccinations [24]. Even though patients with chronic liver disease are at a high risk of morbidity and mortality from COVID-19 [2], there are few studies that have assessed the efficacy of SARS-CoV-2 vaccination in patients with HS. Wang et al. [9] has reported a reassuring result of 95.5% patients with HS showing detectable neutralizing antibody level after two doses of inactivated vaccine. However, there was no control group for comparison, and the vaccine used was an inactivated vaccine (BBIBP-CorV). There was no data on mRNA vaccine and CoronaVac (another inactivated vaccine).
Diagnosis of NAFLD was made by either liver biopsy or clinical findings without proportion specified, and immunogenicity according to the severity of HS was not measured. Moreover, immunogenicity after single dose of vaccine, and longer-term data were not evaluated. In correlation with the recent study, our study further evaluated the effectiveness of CoronaVac as well as BNT162b2 in vaccine immunogenicity with HS. Our study is unique in a few different ways. First, we assessed the vaccine immunogenicity after both the first and second doses of vaccination. Second, we used live virus for analysis which is the gold standard for analysing vaccine humoral response [25], as compared to surrogate virus neutralization test which only correlates well with live virus of 0.7–0.8 [13]. Third, our study recruited a homogenous patient population as HS was defined consistently by CAP measured by transient elastography, as compared to Wang et al.’s study [9] in which HS was identified by either liver biopsy or clinical findings. The proportion of patients undergoing liver biopsy was not specified, and identification of HS from clinical findings alone may lead to potential misclassification of HS status. CAP measurement also allowed us to investigate vaccine immunogenicity according to severity of HS. It is noteworthy that presence of NASH was only identified by raised ALT in our study, although there is no optimal ALT level to predict NASH and advanced fibrosis, and a low normal ALT does not fully exclude the presence of steatohepatitis [26,27].
To our knowledge, our study is the first to compare the immunogenicity of both inactivated virus and mRNA vaccines among patients with and without moderate/severe HS. We found no difference in the seroconversion rate or vMN GMT after either first or second dose between the HS and control groups, regardless of the vaccine platform. Although the level of antibody or neutralizing activity required to confer protection against future infection is currently not well defined, a higher level of antibodies is likely associated with a higher level of protection against future infections [7,28]. To further evaluate the correlation between HS and vaccine immunogenicity, we compared the proportion of highest-tier titer vaccine responders between HS and control groups. It is observed that a lower proportion of HS patients achieved the highest-tier titer response for both BNT162b2 and CoronaVac. Various mechanisms have been proposed, in particular for inactivated vaccine, including links through hyperglycemia, insulin resistance [29], obesity, gut microbiota imbalance [30], and alterations in innate immunity [31]. Nevertheless, the effect of DM and overweight/obesity was adjusted for in the multivariable analysis in our study. Specifically, for BNT162b2, although the seroconversion rate sustained at day180, the vMN GMT decreased at day180, and the significant difference in the proportion of highest-tier titer vaccine response rate between HS and control groups was no longer observed, which is likely due to underpower. This shows the waning effect of vaccine immunogenicity with time. COVID-19 re-infection was reported 3 to 5 months after initial infection due to diminishing serum neutralizing antibody levels [32,33]. A recent study showed progressive waning of vaccine effectiveness against COVID-19 infection among BNT162b2 recipients, from 92% at 15–30 days to 47% at 121–180 days, and subsequently to 23% from day211 onwards [34]. Data on the immunogenicity at day180 for CoronaVac recipients are not available in our study. Further long-term follow-up studies with larger sample size are needed to illustrate the influence of HS on the long-term vaccine immunogenicity.
Regarding safety, COVID-19 vaccines were well tolerated with mild and self-limiting adverse events. There were difference in safety profile after 1st dose and 2nd dose vaccine for different vaccine platforms. After 1st dose vaccine, HS group showed higher rate of systemic adverse events than control group among BNT162b2 recipients. The underlying mechanism was not well understood but it could be related to circulating concentrations of inflammatory cytokines. After 2nd dose vaccine, adverse events were generally similar between HS and control groups, except for the difference in local reactions of CoronaVac recipients between HS and control groups. It could be explained by higher subcutaneous tissue thickness in patients with HS that serve as a protective barrier to localized pain [35].
Several limitations of our study should be noted. First, the sample size was relatively small considering the potential interaction between different vaccine platforms and HS severity. In particular, there were only 14 subjects with moderate/severe HS in CoronaVac recipients. Further study with a larger sample size will allow for better evaluation. Second, although HS was defined by CAP measured by transient elastography, magnetic resonance elastography (MRE) with high diagnostic accuracy of 0.9 was still remained as the most sensitive non-invasive modality in diagnosing HS [36,37]. Third, vaccine-induced cellular immunity against SARS-CoV-2 was not investigated. It is proposed that vaccine-induced T-cell response may protect against severe infection despite seronegativity [7], via suppressing viral replication and producing long-term memory of the immune system [38]. Fourth, data on neutralizing antibody against mutant viruses like Delta or Omicron are not available.
While there was no difference in the seroconversion rate between subjects with or without moderate/severe HS after two doses of either BNT162b2 or CoronaVac, a lower proportion of moderate/severe HS patients achieved a highest-tier humoral response for both types of vaccines. Whether HS is an independent risk factor for poorer vaccine immunogenicity warrants further investigation.
Notes
Authors’ contributions
Drs. Ka-Shing Cheung and Lok-Ka Lam were involved with study concept and design; acquisition of data; analysis and interpretation of data; drafting of manuscript; Drs. Rex Wan Hin Hui, Xianhua Mao, Ruqi R Zhang and Kwok-Hung Chan were involved with analysis and interpretation of data; and critical revision of the manuscript for important intellectual content. Profs. Wai-Kay Seto and Man-Fung Yuen were involved with the study concept and design; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content; and study supervision. The corresponding author had full access to all data, and was fully responsible for the data integrity and statistical analysis. All authors revised the manuscript and approved the final version of this article.
Conflicts of Interest
The authors have no conflicts to disclose.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Neutralizing antibody responses of patients receiving BNT162b2 (n=228)
Adverse events after 1st and 2nd dose of BNT162b2 and CoronaVac
Abbreviations
ALT
alanine aminotransferase
aOR
adjusted odds ratio
CAP
controlled attenuation parameter
CI
confidence interval
COVID-19
coronavirus disease 2019
DM
diabetes mellitus
GMT
geometric mean titer
HS
hepatic steatosis
IQR
interquartile range
MAFLD
metabolic-associated fatty liver disease
MRE
magnetic resonance elastography
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
vMN
virus Microneutralization
References
Appendices
Article information Continued
Notes
Study Highlights
• There is no difference in the seroconversion rate of neutralising antibody between patients with and without moderateto-severe hepatic steatosis after two doses of vaccines (for either BNT162b2 or CoronaVac).
• A lower proportion of patients with moderate-to-severe hepatic steatosis achieve a highest-tier humoral response after two doses of vaccines (for either BNT162b2 or CoronaVac).
• There are no serious adverse reactions in COVID-19 vaccine recipients with moderate-to-severe hepatic steatosis.