Low-level viremia and cirrhotic complications in patients with chronic hepatitis B according to adherence to entecavir
Article information
Abstract
Background/Aims
Low-level viremia (LLV) after nucleos(t)ide analog treatment was presented as a possible cause of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB). However, detailed information on patients’ adherence in the real world was lacking. This study aimed to evaluate the effects of LLV on HCC development, mortality, and cirrhotic complications among patients according to their adherence to entecavir (ETV) treatment.
Methods
We performed a retrospective observational analysis of data from 894 consecutive adult patients with treatment-naïve CHB undergoing ETV treatment. LLV was defined according to either persistent or intermittent episodes of <2,000 IU/mL detectable hepatitis B virus DNA during the follow-up period. Good adherence to medication was defined as a cumulative adherence ≥90% per study period.
Results
Without considering adherence in the entire cohort (n=894), multivariate analysis of the HCC incidence showed that LLV was an independent prognostic factor in addition to other traditional risk factors in the entire cohort (P=0.031). Good adherence group comprised 617 patients (69.0%). No significant difference was found between maintained virologic response and LLV groups in terms of the incidence of liver-related death or transplantation, HCC, and hepatic decompensation in good adherence group, according to multivariate analyses.
Conclusions
In patients with treatment-naïve CHB and good adherence to ETV treatment in the real world, LLV during treatment is not a predictive factor for HCC and cirrhotic complications. It may be unnecessary to adjust their antiviral agent for patients with good adherence who experience LLV during ETV treatment.
Graphical Abstract
INTRODUCTION
Effective antiviral nucleos(t)ide analog (NA) treatment for chronic hepatitis B (CHB) using potent drugs with a high genetic barrier, such as entecavir (ETV) or tenofovir disoproxil fumarate (TDF), has been shown to regress hepatic fibrosis, prevent liverrelated complications, and improve patient survival [1-6]. However, the risk of hepatic complications, particularly the development of hepatocellular carcinoma (HCC), in CHB was not fully eliminated, even with potent agents [7-11]. Low-level viremia (LLV) has been suggested as a possible cause of HCC in patients receiving NA treatment [12,13]. According to Kim et al. [13], LLV, defined as a lack of maintained virologic response (MVR) during ETV monotherapy, was associated with a higher risk of HCC, especially in those with cirrhosis. However, the recently updated American Association for the Study of the Liver Disease guidelines recommend that patients with LLV who are on ETV or TDF monotherapy continue monotherapy [14]. Furthermore, nonadherence to NA treatment may lead to treatment failure. Adherence to treatment refers to the extent to which a patient takes medication as prescribed and for the duration of treatment agreed upon by the patient and physician [15,16]. In real-world clinical settings, nonadherence is common and can restrict the full benefits of antiviral treatment [17-19]. In a previous study, poor treatment adherence was demonstrated to be a significant risk factor for mortality, HCC, and hepatic decompensation [20]. Treatment nonadherence is likely to be a more significant contributor to treatment failure than antiviral resistance against anti-hepatitis B virus (HBV) agents, such as ETV, which exhibit potent viral suppression with a lower risk of drug resistance [18,19]. This study aimed to evaluate the effect of LLV on HCC development, mortality, and cirrhotic complications in patients with CHB according to their adherence to ETV medication in real-world clinical settings.
PATIENTS AND METHODS
Patients
In this retrospective longitudinal observational study, we used the electronic medical records of patients with treatment-naïve CHB who received ETV therapy between January 1, 2007 and January 31, 2017 at Ulsan University Hospital, a tertiary referral center in South Korea. Patients were eligible for inclusion at the time of the initiation of antiviral treatment if they met the following criteria: age ≥18 years; hepatitis B surface antigen (HBsAg)-positive for >6 months or CHB defined by clinical history; no malignancy including HCC at baseline; no evidence of viral coinfections (i.e., human immunodeficiency virus, hepatitis C virus, or hepatitis D virus); no alcohol-related liver disease or autoimmune hepatitis; and treatment-naïve prior to starting ETV 0.5 mg per day (ETV has been available in South Korea since January 1, 2007). The exclusion criteria included the following: follow-up duration <1 year; evidence of decompensated cirrhosis as indicated by the presence (or history) of ascites, esophageal, or gastric variceal bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatorenal syndrome, liver transplantation, or a Child-Pugh score ≥7; serum creatinine level >1.5 mg/dL at baseline; HBV DNA <2,000 IU/L at baseline; or death within 6 months of treatment initiation. Patients who developed HCC within the first year of enrollment were also excluded to minimize the inclusion of pre-existing unidentified HCC and the misattribution of treatment effect. We also excluded patients with renal disease who needed dose adjustments that could affect the adherence rate. Finally, 894 patients were enrolled. Information about baseline patient characteristics and clinical outcomes were obtained from complete inpatient and outpatient medical records. This cohort was originally investigated by Shin et al. [20], and clinical progress was further updated through August 31, 2018 for the current study. This study was approved by the Institutional Review Board of Ulsan University Hospital (#IRB No. 06-2017-26). The requirement for informed consent was waived, as patient records and information were de-identified prior to analysis.
Serum assay
Whole blood count, biochemical and HBV virologic markers, and serum HBV DNA levels were assessed for all patients every 3–6 months during ETV therapy. Serum HBV DNA levels were quantified using the COBAS® TaqMan HBV test (Roche, Branchburg, NJ, USA), which has a lower detection limit of 12 IU/mL (60 copies/mL). Levels of HBsAg, hepatitis B e-antigen (HBeAg), and antibodies to hepatitis B e-antigen (anti-HBe) were examined using an enzyme immunoassay. The virologic outcomes included virologic response (VR), virologic breakthrough (VBT), and MVR or LLV during the on-treatment follow-up period. VR was defined as undetectable serum HBV DNA by polymerase chain reaction assay (<12 IU/mL) for two consecutive measurements during ETV treatment. VBT was defined as an increase of >1 log10 IU/mL in serum HBV DNA level from nadir for two consecutive measurements or on the last available measurement. Based on the serum HBV DNA levels during the follow-up period, patients were categorized as either MVR or LLV at the last follow-up, as mentioned in a previous study by Kim et al. [13] MVR was defined as having serum HBV DNA persistently undetectable throughout the follow-up period after achieving a VR. The remaining patients showed either persistent or intermittent episodes of detectable serum HBV DNA <2,000 IU/mL during follow-up, which was defined as LLV. Restriction fragment mass polymorphism (RFMP; Genematrix®, Youngin, Korea) was used to identify ETV-resistant mutations in the HBV polymerase gene following VBT occurrence during the treatment period. The RFMP assay can detect 100 copies of HBV genome/mL [21]. For this study, we have further investigated and updated the history of diabetes mellitus (DM) and hypertension. DM was defined as a self-reported history of diabetes, use of antidiabetic medication, and/or those with a fasting plasma glucose ≥126 mg/dL. Hypertension was defined as blood pressure ≥140/90 mmHg or a self-reported history of hypertension and/or use of anti-hypertensive medication.
Follow-up assessments
During the follow-up period, all patients underwent periodic surveillance with ultrasonography and laboratory workups, including α-fetoprotein and protein-induced vitamin K absence or antagonist-II, every 6 months to screen for HCC. Liver cirrhosis was clinically defined based on repeated liver imaging studies (nodular liver surface or caudate lobe hypertrophy) with thrombocytopenia (<150×1,000/mm3) or splenomegaly (by imaging), and/or by the presence of varices (by upper endoscopy or imaging studies) [22]. HCC diagnosis was confirmed using radiology (dynamic computed tomography and/or magnetic resonance imaging), as recommended by international guidelines [23]. The clinical outcomes were the cumulative incidence of liver-related death or transplantation, HCC, and hepatic decompensation (ascites, variceal bleeding, spontaneous bacterial peritonitis, hepatic encephalopathy, and hepatorenal syndrome). Liver-related death was defined as death related to cirrhotic complications and HCC. To avoid statistical repetition, the earliest cirrhotic complication was selected if a given patient experienced different types of cirrhotic complications at different time points.
Medication adherence
Medical and pharmacy refill records were reviewed to assess medication adherence by prescription records and the actual amount of medication taken by the patient. Adherence rate was expressed as the percentage of days the patient had medications in his/her possession during the period in which he/she was undergoing therapy. This proportion was calculated as the sum of days on which medication was supplied (obtained over a series of intervals) divided by the total treatment duration (days), which was derived from the dates of the first and last prescriptions dispensed. The medication from the final prescription refill was not included, as its consumption was unknown. Good adherence to medication was defined as a cumulative adherence ≥90% per study period among patients who were prescribed ETV in a given period. The cutoff of 90% was previously used in a similar study [20,24].
Statistical analyses
Continuous variables were compared using the Student’s t-test or Mann-Whitney U-test, and categorical variables were compared using the chi-square test or Fisher’s exact test. Serum HBV DNA (IU/mL) levels were logarithmically transformed for analyses. All statistical tests were considered significant with two-sided P-values of <0.05. Cumulative probabilities of the clinical outcomes were calculated using the Kaplan-Meier method. Univariate and multivariate analyses for factors predictive of the development of HCC were performed using a Cox proportional-hazard model. The results of the model were presented as a hazard ratio (HR) with a 95% confidence interval (CI). Variables were not included in the multivariate model if P-values in the univariate analysis were >0.2. Statistical analyses were performed using the statistical package SPSS for Windows (version 24.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics and virologic outcomes in the entire cohort
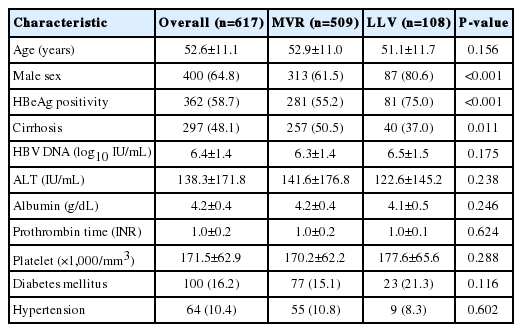
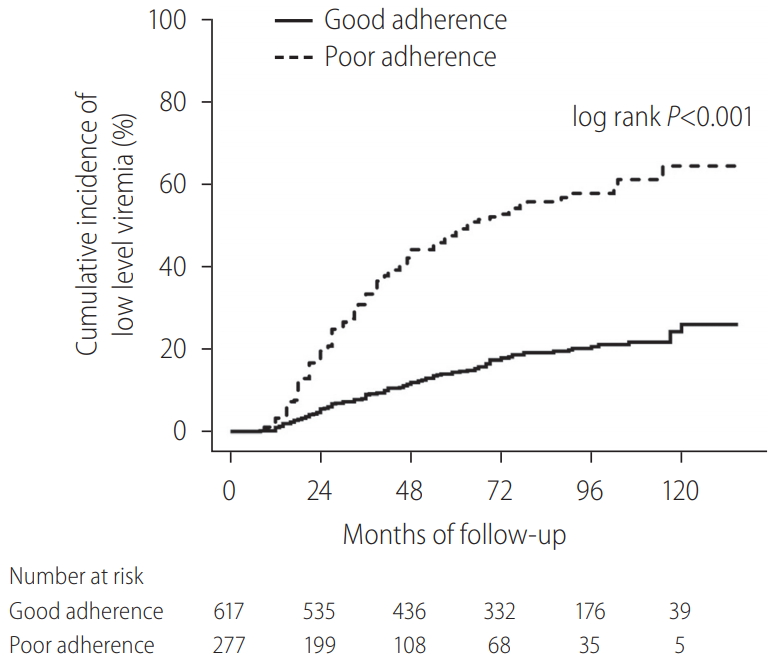
Overall, 1,955 patients with NA-naïve CHB were consecutively treated with ETV during the study period from January 1, 2007 to August 31, 2018. Among these patients, 894 were considered eligible for analysis (Supplementary Fig. 1). The demographic and clinical characteristics of all patients included in this study are summarized in Table 1. VR was observed in 812 patients (90.8%). The median time to VR was 0.8 years (range, 0.1–6.6). The cumulative VR incidence rates were 66.3%, 85.2%, and 95.2% at 1, 3, and 5 years, respectively. Among the patients, MVR was achieved in 654 (73.2%), and LLV remained in 240 (26.8%). During follow-up, 125 patients (14.0%) experienced VBT (good adherence group, n=30; poor adherence group, n=95), with lower rates in good adherence group than in poor adherence group (4.9% vs. 34.3%, P<0.001). The cumulative probability of VBT was 0.6%, 6.7%, 10.8%, and 12.4% at years 1, 3, 5, and 7, respectively. Good adherence to ETV medication was observed in 617 patients (69.0%), and 277 patients (31.0%) had poor adherence. Among the patients with good adherence, MVR was achieved in 509 (82.5%), and LLV remained in 108 (17.5%) (Table 2). Kaplan-Meier survival analysis demonstrated a significantly higher risk of LLV in poor adherence group than in good adherence group (log-rank P<0.001; Fig. 1). There were a total of 42 cases of HBV DNA ≥2,000 IU/mL in 125 VBT patients. Among them, 17 patients (including 11 patients with ETV-resistant mutations) were eventually replaced by TDF, at which point the follow-up for the study was censored. For the remaining 25 patients, at HBV DNA ≥2,000 IU/mL, the compliance was investigated to encourage ETV medication. As a result, within 3 months (next follow-up test) for 23 and within 6 months for two patients, HBV DNA was reduced to below 2,000 IU/mL, in which case ETV treatment was continued. The 25 patients who continued to take ETV even though HBV DNA was ≥2,000 IU/mL did not show ≥2,000 IU/mL again in their subsequent observations, and all of them were considered LLV in this analysis. Among the patients with LLV, 80 underwent mutation tests and 22 developed ETV-resistant mutations (L180M/M204V/S202G [n=13]; L180M/M204V/T184LSM [n=5]; L180M/M204V/M250L [n=4]). The cumulative probability of acquiring a genotypic ETV mutation was 0%, 1.5%, 2.7%, and 3.5% at years 1, 3, 5, and 7, respectively. Regarding LLV, 56/240 patients received TDF, and for statistical analysis, those patients were censored after they switched from ETV to TDF. Of the 56 TDF replacement patients, 45 were caused by VBT and 11 were non-VR cases (ETV-resistant mutations were existed in all non-VR cases).
Clinical outcomes of the entire cohort
The clinical outcomes of the entire cohort, according to MVR vs. LLV, are shown in Figure 2. During the follow-up period of up to 132 months, HCC developed in 91/894 patients (10.2%): 58/654 (8.7%) in MVR group and 33/240 (13.8%) in LLV group. The cumulative incidence of HCC was significantly lower in MVR group than in LLV group: 5- and 10-year cumulative incidence rates of HCC in MVR vs. LLV were 7.8 vs. 11.5% and 12.8 vs. 20.1%, respectively (log-rank P=0.017). Liver-related death or transplantation occurred in 22/894 patients (2.5%) (14/654 [2.1%] in MVR group and 8/240 [3.3%] in LLV group), and hepatic decompensation occurred in 44/894 patients (4.9%) (27/654 [4.1%] in MVR group and 17/240 [7.1%] in LLV group). However, in terms of other clinical outcomes, no statistically significant difference was found between MVR and LLV groups (log-rank P=0.245 for liver-related death or transplantation; log-rank P=0.050 for hepatic decompensation).
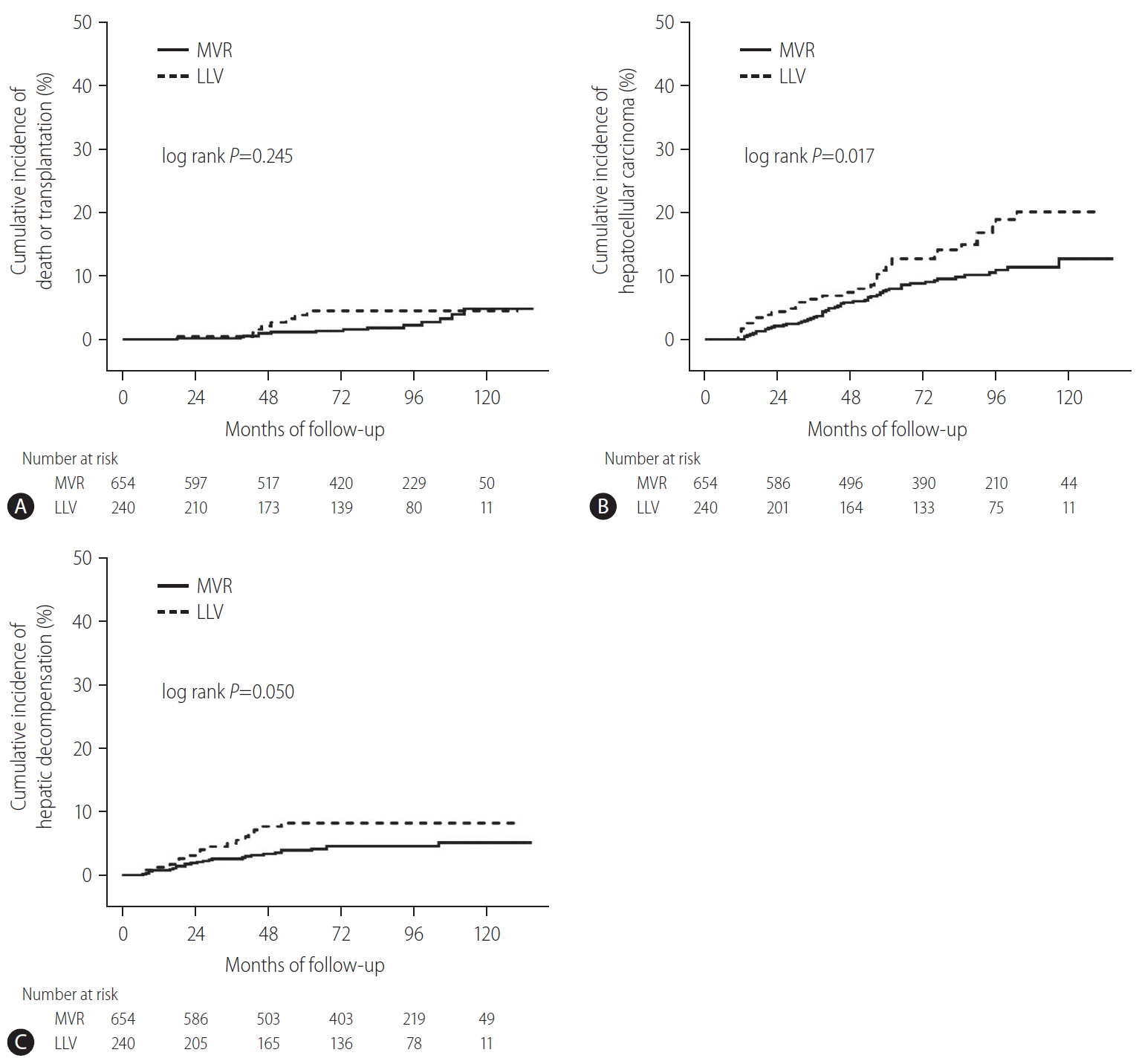
Cumulative incidence of liver-related death or transplantation, HCC, and hepatic decompensation in the entire cohort (n=894). (A) Cumulative incidence of liver-related death or transplantation according to MVR vs. LLV. (B) Cumulative incidence of HCC according to MVR vs. LLV. (C) Cumulative incidence of hepatic decompensation according to MVR vs. LLV. MVR, maintained virologic response; LLV, low-level viremia; HCC, hepatocellular carcinoma.
Clinical outcomes of good adherence group
Clinical outcomes according to MVR vs. LLV in good adherence group are shown in Figure 3. Overall, during the follow-up period of up to 132 months, HCC developed in 43/617 patients (7.0%): 36/509 (7.1%) in MVR group and 7/108 (6.5%) in LLV group. Liver-related death or transplantation occurred in 4/617 patients (0.6%) (4/509 [7.9%] in MVR group and 0/108 [0.0%] in LLV group), and hepatic decompensation occurred in 12/617 (1.9%) patients (12/509 [2.4%] in MVR group and 0/108 [0.0%] in LLV group). The cumulative incidence of liver-related death or transplantation, HCC, and hepatic decompensation showed no significant differences between MVR and LLV groups (log-rank P=0.359 for liver-related death or transplantation; log-rank P=0.937 for HCC; log-rank P=0.118 for hepatic decompensation). The development of HCC also showed no difference between MVR and LLV groups in the subgroup analysis regarding cirrhotic subcohort (log-rank P=0.536) (Supplementary Fig. 2).

Cumulative incidence of liver-related death or transplantation, HCC, and hepatic decompensation in good adherence group (n=617). (A) Cumulative incidence of liver-related death or transplantation according to MVR vs. LLV. (B) Cumulative incidence of HCC according to MVR vs. LLV. (C) Cumulative incidence of hepatic decompensation according to MVR vs. LLV. MVR, maintained virologic response; LLV, low-level viremia; HCC, hepatocellular carcinoma.
Risk factors for HCC
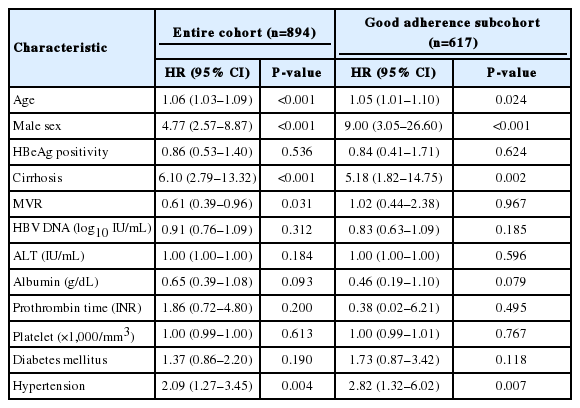
Multivariate analyses were performed to compare the effect of LLV to other well-known risk factors for the development of HCC in the entire cohort and good adherence group (Table 3). Multivariate analysis showed that among the entire cohort, in addition to the traditional risk factors for HCC such as old age, male sex, and cirrhosis, MVR presented a statistically significant association with the development of HCC with a HR of 0.61 (95% CI, 0.39–0.96; P=0.031). However, in the analyses of good adherence group, LLV showed no independent association with HCC (P=0.967), whereas the traditional risk factors for HCC, such as age, male sex, and cirrhosis, presented strong associations with the development of HCC in multivariate analysis.
DISCUSSION
According to multivariate analysis, LLV during treatment was not a predictive factor for HCC and cirrhotic complications in patients with CHB and good adherence to ETV treatment. With the development of antiviral agents, a dramatic change has occurred in the treatment and prognosis of CHB. In particular, potent antiviral agents with high genetic barriers enabled continued use of medication and showed long-term results in reduced mortality and the progression of cirrhosis or HCC [1-6]. However, even with the prolonged use of antiviral agents, complications of CHB, particularly HCC, are not fully inhibited [7-11]. A multicenter study which investigated the incidence of HCC in 1,666 Caucasian patients with CHB receiving ETV or TDF showed that the cumulative probability of HCC was 3.4% at year 3 and 8.7% at year 5, with an annual incidence rate of 1.37% per year [25]. The annual incidence of HCC in Caucasian patients with compensated HBV-related cirrhosis was about 2.2%, and the 5-year cumulative incidence was about 10% [26]. Previous studies of Korean patients showed that even with optimal VR under NA therapy, the annual incidence of HCC was 0.15–0.80% per year in non-cirrhotic patients, and 0.95–3.55% per year in compensated-cirrhotic patients [10,27,28].
LLV has been suggested as a possible cause of HCC in patients receiving NA treatment [12,13]. A previous study showed that LLV, defined as a lack of MVR, significantly increases the incidence of HCC in patients with CHB who are receiving ETV [13]. Sub-optimally suppressed viral replication in the liver can cause continuous inflammation, and the proliferation of inserted HBV oncogenes is also considered an important mechanism of liver cancer [29-31]. In a previous study, patients with LLV in whom a continuous viral response was not achieved had an increased cumulative incidence of HCC, which was more noticeable in patients with cirrhosis [13]. This was also evident in our study; subgroup analysis of the cirrhotic subcohort showed that the cumulative incidence of HCC and hepatic decompensation were significantly lower in MVR group than in LLV group (log-rank P=0.001 and log-rank P=0.011, respectively) (Supplementary Fig. 3).
Meanwhile, in another study, we presented poor treatment adherence as a significant risk factor for mortality, HCC, and hepatic decompensation [20]. Data on adherence to NA treatment for CHB are limited. In a previous study, a pharmacy claims database with 11,100 patients receiving NAs for CHB was used to investigate the rates of patient adherence in a real-world setting [32]. The overall adherence rates were found to be 87.8%, with 55.3% of patients having ≥90% adherence. Other studies have reported mean NA treatment adherence rates ranging from 81% to 99% [33,34]. This was consistent with the mean adherence rate of 89.1% observed in the current study [20]. Despite the significance of viral suppression to prevent cirrhotic complications and mortality, evidence on the association between adherence to ETV treatment and hepatic complications still remain limited. In our previous study, we evaluated the association of adherence to ETV treatment on hepatic complications in patients with CHB [20]. Multivariate Cox proportional regression analyses demonstrated that adherence to ETV treatment was a powerful factor for HCC development, cirrhotic complications, and liver-related or all-cause mortality. In the same study, continuous viral response and MVR increased, whereas VBT was significantly low in patients with good adherence, indicating the correlation between adherence and viral response. These results suggest that poor adherence to antiviral agents is closely linked to LLV. In our current study, when we assessed the correlation between LLV and adherence, LLV was significantly lower in patients with good adherence (Fig. 2). Accordingly, we intended to evaluate the impact of LLV on the risk of HCC, rate of hepatic decompensation, and survival rate in this study in good adherence group and the entire cohort.
According to Kim et al. [13], MVR was achieved in 498/875 patients (56.9%) in the study population, and HCC developed in 85/875 patients (9.7%) within 60 months; these figures possibly included a significant number of patients with poor adherence. Without considering adherence in our entire cohort (n=894), MVR was achieved in 654/894 patients (73.2%), and HCC developed in 91/894 patients (10.2%). This finding was similar to that in a previous report which assessed MVR in 1,466 ETV-treated patients and reported an MVR rate of 78% in patients with cirrhosis and 77% in patients without cirrhosis [35]. This study evaluated LLV as a risk factor for mortality, HCC, and hepatic decompensation in patients with CHB and good adherence to ETV. The results of this study showed that, without considering adherence, patients with CHB and LLV had a significantly increased incidence of HCC, in addition to other traditional risk factors in the entire cohort. On the other hand, no significant difference was found in the clinical outcomes between MVR and LLV groups among good adherence group in our study, and these results were found in poor adherence group as well (Supplementary Table 1).
To directly compare the effect of LLV and medication adherence on the development of HCC, we performed univariate and multivariate analyses (Supplementary Table 2); in addition to the traditional risk factors for HCC, such as old age, male sex, and cirrhosis, good adherence to ETV treatment was highly associated with the development of HCC with a HR of 0.40 (95% CI, 0.25–0.66; P<0.001). However, MVR was not independently associated with HCC (P=0.361). Notably, in the case of multivariate analyses not including adherence (Supplementary Table 3), MVR was significantly associated with the development of HCC with a HR of 0.61 (95% CI, 0.39–0.96; P=0.031), in addition to the other risk factors: old age, male sex, and cirrhosis. On the other hand, among patients with MVR (n=654), HCC developed in 36/509 patients (7.1%) in good adherence group and 22/145 (15.2%) in poor adherence group during the follow-up period of up to 132 months. Kaplan-Meier survival analysis demonstrated a significantly higher risk of HCC in poor adherence group compared to good adherence group (log-rank P<0.001). Liver-related death or transplantation occurred in 4/509 (0.8%) in good adherence group and 10/145 (6.9%) in poor adherence group. Hepatic decompensation occurred in 12/509 (2.4%) in good adherence group and 15/145 (10.3%) in poor adherence group. Kaplan-Meier survival analysis demonstrated a significantly higher risk of liver-related death or transplantation, as well as hepatic decompensation, in poor adherence group compared to good adherence group (log-rank P<0.001).
In this study, adherence was shown to be a more significant factor than LLV for predicting the occurrence of cirrhotic complications. However, MVR vs. LLV and good vs. poor adherence are not necessarily different concepts, and both are indicators that reflect the intra-hepatic viral replication of HBV. In the statistics of our study, the result that LLV does not appear to be an independent risk factor for HCC occurrence does not infer that LLV is not related to HCC occurrence, but is rather a confounding factor closely related to adherence. The ideal viral suppression is a key mechanism to prevent cirrhotic complications in patients with CHB, such as HCC. In general, LLV is based on a serologic test for HBV DNA conducted at 3-to-6-month intervals, with the limitation that viral replication status in the middle of testing is unknown. In contrast, nonadherence refers to a period of inadequate medication during follow-up, suggesting the possibility of viral proliferation during the period of nonadherence. In other words, as sustained antiviral therapy is important for HBV inhibition, theoretically, adherence can be considered as a continuous variable that more accurately reflects intra-hepatic viral loads compared to LLV with periodic serologic testing.
A previous study of Korean patients with optimal VR during NA therapy showed that the cumulative HCC incidence was 8.1% at 3 years and 17.4% at 5 years with baseline liver cirrhosis, and 4.7% at 3 years and 7.2% at 5 years without baseline liver cirrhosis [9]. In our study, the 5- and 10-year cumulative incidence rates of HCC in good adherence vs. poor adherence were 5.8 vs. 15.9% and 10.3 vs. 26.0%, respectively, in the entire cohort (Supplementary Fig. 4). In terms of cirrhotic subcohort, the 5- and 10-year cumulative incidence rates of HCC in good adherence vs. poor adherence were 10.0 vs. 28.9% and 17.1 vs. 44.1%, respectively (Supplementary Fig. 5). These results suggest that the long-term preventive effects of ETV treatment may be significantly attenuated in patients with poor adherence. This again presents the importance of continuous and regular medication in patients with CHB.
The strengths of this study were its large sample size and long-term follow-up, which together allowed for increased statistical power and greater reliability of data. We also sought to analyze from multiple perspectives to clarify the relationship between adherence, LLV, and cirrhotic complications. Nonetheless, this study had some limitations. First, the retrospective nature of certain clinical information might be biased due to incomplete data collection. The common reasons affecting patient adherence, such as lack of recognition, forgetfulness, overriding priorities, and emotional or cultural factors, may be expected to occur in patients prescribed NA therapy. However, due to the retrospective nature of this study, the causes could not be identified in approximately 30% of the patients. Second, individuals who developed LLV had different durations of LLV during the follow-up period, which may have had different degrees of impact on clinical outcomes. Furthermore, defining LLV or MVR is subject to the frequency and intervals of HBV DNA testing and may not be the same for all patients, which may result in classification bias. Third, since the number of patients with cirrhosis and LLV despite good adherence to treatment was very low (n=40, only six cases of HCC), it was too statistically low-powered to draw a definite conclusion regarding the effect of LLV on clinical outcomes among the cirrhotic subgroup. Fourth, since our database did not include other NAs (lamivudine, adefovir dipivoxil, telbivudine, and TDF) besides ETV, the application of these results to other NAs should be considered with caution. Finally, although pharmacy refill records are objective measures and are routinely collected, they do not measure whether the participants actually took their medications. The rates of nonadherence may have been overestimated if the dispensed medications were not used. In addition, identifying and understanding the factors influencing nonadherence to medication is necessary to identify appropriate interventions.
In conclusion, no significant difference was found between MVR and LLV groups in terms of the incidence of liver-related death or transplantation, HCC, and hepatic decompensation in patients with good adherence. For patients with suboptimal virological response, it is helpful to check their adherence before considering to continue, switch, or add another drug. For patients with good adherence who experience LLV during ETV treatment, it may be unnecessary to adjust their antiviral agent immediately, and careful observation would be possible.
Notes
Authors’ contribution
Study coordination and design, data collection, data analysis, statistical analysis, writing and revision of the manuscript, and approval of the final version: SBL, BRP, and EJP. Study coordination and design, data analysis, critical review of the manuscript, and approval of the final version: NHP and JWS. Data supply, critical review of the manuscript, and approval of the final version: JJ, JHP, SWJ, IDJ, and SJB. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest: The authors have no conflicts to disclose.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Overview of the study population. HCC, hepatocellular carcinoma; ETV, entecavir; HBV, hepatitis B virus.
Cumulative incidence of HCC according to MVR vs. LLV in good adherence group, cirrhotic subcohort (n=297). MVR, maintained virologic response; LLV, low-level viremia; HCC, hepatocelluar carcinoma.
Cumulative incidence of liver-related death or transplantation, HCC, and hepatic decompensation in cirrhotic subcohort (n=440). (A) Cumulative incidence of liver-related death or transplantation according to MVR vs. LLV. (B) Cumulative incidence of HCC according to MVR vs. LLV. (C) Cumulative incidence of hepatic decompensation according to MVR vs. LLV. MVR, maintained virologic response; LLV, low-level viremia; HCC, hepatocellular carcinoma.
Cumulative incidence of liver-related death or transplantation, HCC, and hepatic decompensation in the entire cohort (n=894). (A) Cumulative incidence of liver-related death or transplantation according to good adherence vs. poor adherence. (B) Cumulative incidence of HCC according to good adherence vs. poor adherence. (C) Cumulative incidence of hepatic decompensation according to good adherence vs. poor adherence. HCC, hepatocellular carcinoma.
Cumulative incidence of liver-related death or transplantation, HCC, and hepatic decompensation in cirrhotic subcohort (n=440). (A) Cumulative incidence of liver-related death or transplantation according to good adherence vs. poor adherence. (B) Cumulative incidence of HCC according to good adherence vs. poor adherence. (C) Cumulative incidence of hepatic decompensation according to good adherence vs. poor adherence. HCC, hepatocellular carcinoma.
Multivariate analysis of potential risk factors for hepatocellular carcinoma in the entire cohort and poor adherence subcohort
Univariate and multivariate analyses of potential risk factors for hepatocellular carcinoma
Univariate and multivariate analyses of potential risk factors for hepatocellular carcinoma (not including medication adherence)
Abbreviations
anti-HBe
antibodies to hepatitis B e antigen
CHB
chronic hepatitis B
DM
diabetes mellitus
ETV
entecavir
HBeAg
hepatitis B e antigen
HBsAg
hepatitis Bsurface antigen
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HR
hazardratio
LLV
low-level viremia
MVR
maintained virologic response
NA
nucleos(t)ide analog
RFMP
restriction fragment mass polymorphism
TDF
tenofovirdisoproxil fumarate
VBT
virologic breakthrough
VR
virologic response
References
Article information Continued
Notes
Study Highlights
Nonadherence to medication and low-level viremia (LLV) can restrict the full benefits of antiviral treatment for chronic hepatitis B.
In patients with good adherence (≥90%) to entecavir treatment in the real-world, LLV during treatment was not a risk factor for hepatocellular carcinoma and cirrhotic complications.
It is helpful to check the adherence of the patient with a suboptimal virological response, prior to considering adjusting their antiviral agent immediately and for patients with good adherence, it may be unnecessary and careful observation will be possible.