KASL clinical practice guidelines for liver cirrhosis: Ascites and related complications
Article information
PREAMBLE
Aims
Ascites is one of the most common complications of liver cirrhosis along with variceal bleeding and hepatic encephalopathy. It is often the first sign of decompensated cirrhosis with portal hypertension. Patients with compensated cirrhosis progress to decompensated cirrhosis at a rate of 5-7% per year, and about 50% of the cases develop ascites within 10 years after diagnosis of liver cirrhosis. The 1-year and 2-year survival rates of patients with decompensated cirrhosis complicated with ascites are 60% and 45%, respectively, which is significantly lower than the 1-year and 2-year survival rates (95% and 90%) of patients with compensated cirrhosis [1,2].
According to the National Statistical Office, the mortality rate due to liver disease was 13.4 per 100,000 persons in 2015, the eighth highest cause of death in Korea. It has declined compared to 2005 (mortality rate due to liver disease was 17.2 per 100,000 persons, the sixth highest cause of death cause in Korea). Liver cirrhosis and hepatocellular carcinoma (HCC) are a major cause of death in patients with chronic liver disease. Korean Association for the Study of the Liver (KASL) published guidelines for the management of liver cirrhosis in 2005 which proposed guidelines for the treatment of major complications of liver cirrhosis, including ascites, hepatorenal syndrome, varices and hepatic encephalopathy. In 2011, the guidelines for the management of liver cirrhosis were revised to cover diagnosis of liver cirrhosis, anti-fibrotic treatment of cirrhosis, variceal bleeding, ascites, and hepatic encephalopathy. Six years after the publication of the 2011 guidelines, the need arose to revise the guidelines for liver cirrhosis based on new evidence accumulated. Therefore, KASL revised the guidelines for ascites, a major complication of liver cirrhosis. The revisions were based on a systematic approach that reflects evidence-based medicine and expert opinions. This guideline is intended to be used as a practical reference for the treatment of cirrhotic patients with ascites and related complications, and they do not represent an absolute standard of care. The best choice for each patient’s care will vary from case to case, and the judgment of the treating physician is important. This guideline may change when medical evidence based on new research findings is accumulated in the future.
Target population
The guideline targets patients with ascites and related complications (e.g. refractory ascites, spontaneous bacterial peritonitis, hyponatremia, acute kidney injury, hepatorenal syndrome) due to liver cirrhosis. The guideline is intended for clinicians and medical personnels who are in charge of the diagnosis and treatment of patients with liver cirrhosis. This guideline also intended to provide useful clinical information and directions for resident physicians and fellows in training, practitioners, and trainers.
The development, funding, and revision process
Comprising 10 hepatologists, The Clinical Practice Guideline Committee for Liver Cirrhosis: Ascites and Related Complications (‘The Committee’) was organized according to the proposal and approval of the KASL Board of Executives. Funding for the revisions was provided by KASL. Each committee member collected and analyzed source data in his or her own field, and the members then wrote the manuscript together.
Literature review
The Committee systematically collected and reviewed international and domestic literature published in PubMed, MEDLINE, KoreaMed, and other databases. In addition to published articles, abstracts of important meetings published before August 2017 were evaluated. Key words and key questions were selected using PICO (Patient/Problem, Intervention, Comparison, Outcome) assessments.
Levels of evidence and grades of recommendation
The evidence and recommendations were graded according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system, with minor modifications (Table 1) [3]. Levels of evidence were determined based on the possibility of change in a results or clinical outcome after further research. They were described as high (A), moderate (B), or low (C), and were characterized as follows: A, the highest level of evidence with the smallest possibility of change in the conclusion; B, a moderate level of potential change; and C, the lowest level of evidence with the greatest possibility of change. The strength of a recommendation was also classified according to the GRADE system. Each study was classified as a strong recommendation (1) or a weak recommendation (2), based on the quality of evidence, the balance between the desirable and undesirable effect of an intervention, and socioeconomic aspects (including cost and availability). Each recommendation was ultimately graded as A1, A2, B1, B2, C1, or C2, thereby combining the level of evidence (A-C) and the strength of the recommendation (1 or 2).
List of key questions
The Committee selected the following key questions as key components to be covered in this guideline.
1. How to diagnose ascites due to liver cirrhosis?
2. How to treat ascites due to liver cirrhosis?
3. How to manage the nutrition of patients with liver cirrhosis and ascites?
4. How to diagnose and treat refractory ascites?
5. How to treat hyponatremia related to liver cirrhosis?
6. How to diagnose spontaneous bacterial peritonitis?
7. How to treat community-acquired spontaneous bacterial peritonitis?
8. How to treat hospital-acquired spontaneous bacterial peritonitis?
9. How to diagnose acute kidney injury and hepatorenal syndrome in patients with liver cirrhosis?
10. How to treat acute kidney injury and hepatorenal syndrome in patients with liver cirrhosis?
11. How to treat hepatic hydrothorax and abdominal hernia?
12. What should be considered when using drugs in patients with liver cirrhosis?
Review of the manuscript and approval process
Each manuscript written by members was reviewed and approved through meetings of the Committee. The quality of each manuscript and the academic integrity of the contents were evaluated based on the standards suggested by AGREE II (Appraisal of Guidelines for Research and Evaluation II). The guidelines were reviewed at a meeting of an external review board composed of seven KASL members. The guideline was further modified following opinions aired at a public hearing and at a symposium open to all KASL members. The final manuscript was approved by the KASL Board of Executives.
Release of the guidelines and a plan for updates
The revised guideline (The KASL Clinical Practice Guidelines for Liver Cirrhosis: Ascites and Related Complications) was released at a KASL meeting on 23 November 2017. The Korean version of the guideline is available on the KASL website (http://www.kasl.org). Future revisions will be conducted when necessary for the promotion of health in South Korea, following an accumulation of research on the management of ascites and related complications.
ASCITES DUE TO CIRRHOSIS
Diagnosis
History
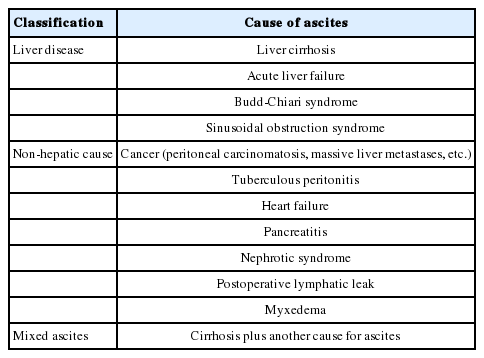
Approximately 75-85% of patients presenting with ascites in foreign countries [4-6], and 60% in a Korean single center [7], have been reported to have liver cirrhosis as the underlying cause. Ascites is also caused by malignancy, tuberculosis, heart failure, pancreatic disease, and nephrotic syndrome (Table 2). Therefore, the initial diagnosis of ascites needs careful examination for differential diagnosis.
Physical examination
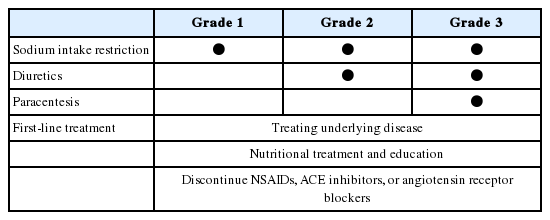
When there is abdomen swelling, it should lead to percussion of the flanks. One should perform shifting dullness and fluid wave tests. The fluid wave test is inconvenient and performs less well than the shifting dullness test [8]. Generally 1,500 mL of fluid must be present before flank dullness is detected. If there is no flank dullness, the patient has less than a 10% chance of having ascites [8]. The physical examination for finding ascites in the obese patient is difficult. An abdominal ultrasound can be helpful to confirm ascites. An abdominal ultrasound can detect ascites only when it exist over 100 mL [9]. Ascites is classified as Grade 1, 2, and 3 according to the amount of ascites. Grade 1 is detected only by imaging techniques, including abdominal sonography. Grade 2 ascites is easily identified by visual inspection and palpation. Grade 3 ascites shows profound distension of the abdomen, as in massive or tense ascites. Patients with heart failure can develop ascites where jugular venous distension is present. Evaluation of blood concentrations of brain natriuretic peptide or pro-brain natriuretic peptide can help discriminate between ascites from heart failure and ascites from cirrhosis [10].
Abdominal paracentesis
Abdominal paracentesis with ascitic fluid analysis is the most rapid and efficient test to diagnose ascites [11,12]. It can ensure the cause of ascites [4] and the infection [12]. A diagnostic paracentesis should be performed 1) in all patients with new-onset Grade 2 or 3 ascites, 2) in all patients hospitalized for worsening ascites, and 3) with any complication of cirrhosis, including fever, abdominal pain, gastrointestinal bleeding, hepatic encephalopathy, hypotension, or renal insufficiency [13]. Suitable sites for paracentesis include the left or right lower quadrant areas. The left lower quadrant is preferred because of the greater depth of ascites and the thinner abdominal wall [14]. Severe hemorrhage occurs in 0.2-2.2% of puncures [15-17], and death is rare [15-21]. In one study, the death rate was 0.02% among 4,729 prodedures [17]. Hemorrhage following paracentesis can occur from the direct puncture of a superficial abdominal wall vein (such as the superficial epigastric vein), of mesenteric varices, or of intraperitoneal collateral vessels (including the paraumbilical vein) [15,19,22]. Bleeding can also appear from a direct puncture of the inferior epigastric artery or the deep circumflex iliac artery [23,24]. Although most reports detected symptoms during the first 6 to 24 h after paracentesis, delayed symptoms up to 1 week after the procedure have also been described [15,25]. Most bleeding can be handled by medical treatment, such as fluid resuscitation, transfusion, and correction of coagulation disorders. However, transcatheter coil embolization [23] or laparoscopy with vessel ligature [25] should be considered when hemodynamic instability persists despite medical treatment. Alternatively, a transjugular intrahepatic portal-systemic shunt (TIPS) [21] or liver transplantation [15] can be considered in cases of severe bleeding.
When there is clinically evident hyperfibrinolysis or disseminated intravascular coagulation, paracentesis should be prohibited. Careful attention is needed for patients with severe liver dysfunction and severe renal dysfunction, as risk of complication is higher [17]. Pregnancy, severe intestinal distension, and a history of extensive abdominal surgery are relative contraindications for paracentesis; in these cases, abdominal sonography can be helpful [26]. The routine prophylactic use of fresh frozen plasma or platelets before paracentesis is not generally recommended [27-29]. However, patients with severe coagulopathy require special precautions for bleeding, and some physicians prefer to transfuse blood products (fresh frozen plasma and/or platelets) before paracentesis. Further study is needed to see whether these prophylactic managements are helpful.
Ascitic fluid analysis and differential diagnosis
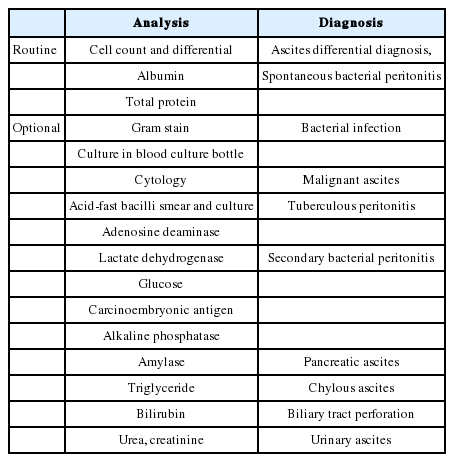
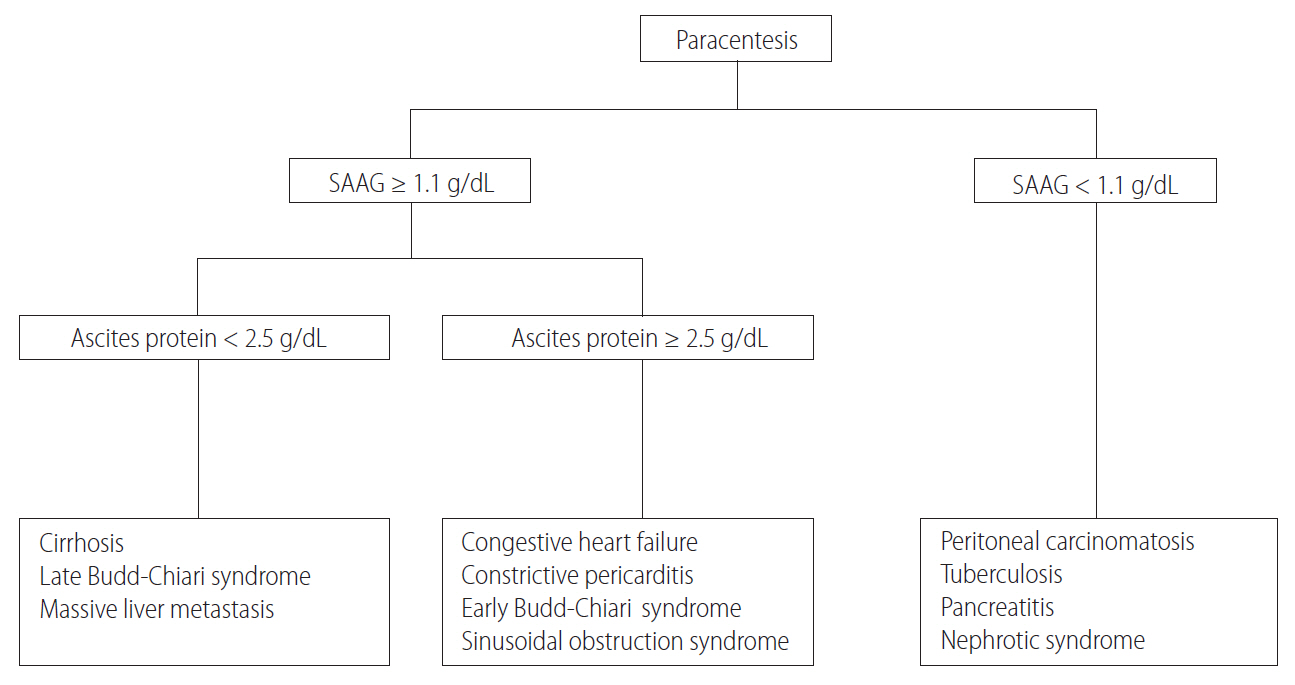
Once ascitic fluid has been extracted, its gross appearance should be examined. Turbid fluid can result from the presence of infection or tumor cells. White, milky fluid indicates a triglyceride level >200 mg/dL (and often >1,000 mg/dL), which is the hallmark of chylous ascites. Chylous ascites results from lymphatic disruption that may occur with trauma, cirrhosis, tumor, tuberculosis, or certain congenital abnormalities. Dark brown fluid can reflect a high bilirubin concentration and indicates biliary tract disruption. Black fluid may indicate the presence of pancreatic necrosis or metastatic melanoma. In uncomplicated ascites due to cirrhosis, screening tests (e.g. cell count and differential, albumin, and total protein concentration) are performed on the initial specimen (Table 3). The serum albumin level should be measured simultaneously to permit calculation of the serum-ascites albumin gradient (SAAG). Calculating the SAAG involves measuring the serum albumin concentration and the ascitic fluid specimens obtained on the same day, and subtracting the ascitic fluid value from the serum value. If the SAAG is greater than or equal to 1.1 g/dL, the patient has portal hypertension, with approximately 97% accuracy [4]. The SAAG is useful for distinguishing ascites caused by portal hypertension from nonportal hypertensive ascites. Possible causes of SAAG values ≥1.1 g/dL include liver cirrhosis, cardiac ascites, hepatic vein thrombosis (Budd-Chiari syndrome), sinusoidal obstruction syndrome (veno-occlusive disease), or massive liver metastases. A SAAG value <1.1 g/dL indicates that the ascites is not related to portal hypertension, and possible causes are tuberculous peritonitis, peritoneal carcinomatosis, or pancreatic ascites (Fig. 1).
Patients undergoing serial therapeutic paracentesis are typically tested only for cell count and differential [30,31]. Repeating tests of total protein and SAAG on fluid removed therapeutically is usually not needed. If ascitic fluid infection is suspected (fever, abdominal pain, or unexplained encephalopathy, acidosis, azotemia, hypotension, or hypothermia), bacterial culture of the fluid in aerobic and anaerobic blood culture bottles inoculated at the bedside should be performed. Additional testing may be performed in each clinical situation (Table 3). When cancer is suspected, ascitic fluid cytology is performed. The ascitic fluid cytology is positive only in the setting of peritoneal carcinomatosis. The sensitivity of cytology in detecting peritoneal carcinomatosis is 96.7% if three samples (from different paracentesis procedures) are sent and processed promptly [32]. Carcinoembryonic antigen (CEA) of the ascitic fluid does not seem to be sensitive enough to diagnose malignancy-related ascites. However, due to its high specificity, high levels of CEA are more likely to be malignancy-related ascites [33]. Tuberculous peritonitis is typically associated with ascitic fluid lymphocytosis but can be difficult to diagnose by paracentesis. When tuberculous peritonitis is suspected, an acid-fast bacilli (AFB) smear, culture, and adenosine deaminase (ADA) assay can be performed. The sensitivity of a smear of ascitic fluid for mycobacteria ranges from 0% to 86% [34-36], and the sensitivity of a fluid culture for mycobacteria ranges from 20% [37] to 57-83% [35,38,39]. In patients with tuberculous peritonitis without cirrhosis, the ADA assay shows a sensitivity of 100% and a specificity of 96.6-100% when the ADA value is higher than 32-40 U/L [40-43]. However, tuberculous peritonitis with cirrhotic ascites yields low total protein in the ascites fluid, and the ADA assay shows low sensitivity [44]. Therefore, patients with liver cirrhosis should carefully rule out a diagnosis of tuberculous peritonitis when they have a low ADA value. In one Korean study of patients with liver cirrhosis, the ADA assay showed a sensitivity of 91.7%, a specificity of 92%, and an accuracy of 91.9% when an ADA cut-off value of 32 U/L was used [45]. A recent study of patients with tuberculous peritonitis with cirrhotic ascites also showed a sensitivity of 100% and a specificity of 93.3% when an ADA cut-off value of 27 U/L was used [46]. These studies indicate that ADA can be useful to diagnose tuberculous peritonitis with cirrhotic ascites. Patients at high risk for tuberculous peritonitis (e.g. recent immigration from an endemic area or acquired immunodeficiency syndrome) should have testing for mycobacteria on the first ascitic fluid specimen [47]. Polymerase chain reaction testing for mycobacteria or laparoscopy with biopsy and mycobacterial culture of tubercles are the most rapid and accurate methods of diagnosing tuberculous peritonitis. When secondary peritonitis resulting from a perforated hollow viscus is suspected, ascitic glucose and lactate dehydrogenase (LDH) levels can be measured. Secondary peritonitis is suggested by an ascitic glucose level <50 mg/dL, or an ascitic LDH level higher than the serum LDH level [48]. An elevation of CEA (>5 ng/mL) or alkaline phosphatase (>240 U/L) can also be helpful for the diagnosis of secondary peritonitis resulting from a perforated hollow viscus [49]. When pancreatic ascites is suspected, the ascitic amylase level should be measured, which is typically >1,000 mg/dL. Rarely, trauma or iatrogenic origin can cause urinary ascites by injury of the bladder or ureter. Elevated levels of urea and creatinine in the ascites fluid can be clues for diagnosis [50]. When the cause of ascites remains uncertain, laparotomy or laparoscopy with peritoneal biopsy for histology and culture remains the gold standard. Approximately 5% of patients with ascites can have two or more causes of ascites formation, including liver cirrhosis, peritoneal carcinomatosis or tuberculous peritonitis (Table 2) [4]. In case of obvious cause for ascites, some cases are finally found to have multiple causes of ascites composition (e.g. heart failure, diabetic nephropathy, and cirrhosis) [51]. In this setting, the sum of predisposing causes makes progress to sodium and water retention, even though each factors might not be enough to cause fluid retention. Patients with ascites (or pleural fluid of any cause) have an elevated serum CA125 level; when ascites is controlled, the CA125 level decreases rapidly [52,53]. CA125 levels are elevated when mesothelial cells are under pressure from the presence of fluid, making this test very nonspecific. CA125 levels is not helpful in the differential diagnosis of ascites. It is not recommended in patients with any type of ascites.
[Recommendations]
1. A diagnostic paracentesis should be performed in all patients with new onset Grade 2 or 3 ascites, in all patients hospitalized for worsening of ascites, and in all patients with any complication of cirrhosis (including fever, abdominal pain, gastrointestinal bleeding, hepatic encephalopathy, hypotension, or renal insufficiency) (A1).
2. The initial laboratory investigation of ascites fluid should include an ascitic fluid cell count and differential, ascitic fluid total protein, and albumin. Calcuation of serum-ascites albumin gradient should be performed for differential diagnosis of ascites (A1).
3. If ascitic fluid infection is suspected, bacterial culture of the fluid in aerobic and anaerobic blood culture bottles inoculated at the bedside should be performed (A1).
Treatment
First-line treatment
Treating underlying disease: The basic treatment for ascites is treatment of the underlying disease. Cirrhotic ascites related to alcohol use, virus hepatitis, or autoimmune liver disease can be controlled by treating the underlying cause of liver disease (Table 4) [54]. Alcoholic cirrhosis is a major cause of ascites [7]. For them, abstinence improves liver fibrosis, lowers portal pressure, and is effective in controlling ascites [55]. Abstinence can lead to the elimination of ascites, increase the response to diuretics, and ultimately, the survival of alcoholic cirrhosis patients with ascites [56]. In a study of patients with alcoholic liver cirrhosis of Child-Pugh class C, the three-year survival rate was approximately 75% for patients who stopped drinking alcohol, but the mortality rate was significantly higher for patients who continued alcohol use [56]. Baclofen acts on GABA receptors and reduce alcohol craving. In a study of alcoholic liver cirrhosis, baclofen use for 5.8 months safely improved bilirubin levels and Model for End-Stage Liver Disease (MELD) scores [57]. In a study of patients with alcoholic liver cirrhosis, 12 weeks of baclofen administration was effective in reducing alcohol craving without adverse effects [58]. In patients with hepatitis B virus-related liver cirrhosis, oral antiviral agents improved liver function and reduced the complications of liver cirrhosis, including ascites [54,59-62]. In a study of 267 patients with hepatitis C virus-related cirrhosis, 12 weeks of treatment with sofosbuvir and velpatasvir improved the MELD score in 51% of patients and improved the Child-Pugh score in 47% of patients [63]. In patients with hepatitis C virus-related cirrhosis and portal hypertension, six patients with pre-treatment ascites had controlled ascites after treatment of sofosbuvir and ribavirin for 24 weeks [64].
Nutritional management and education: Most cirrhotic patients with ascites are malnourished [65]. Depending on the state of the patient, the following carbohydrate, protein, and caloric intakes are recommended: 2-3 g/kg/day carbohydrate, 1.2–1.5 g/kg/day protein, and 35–40 kcal/kg/day caloric intake [66,67]. In the presence of hepatic encephalopathy, administration of a branched-chain amino acid (BCAA) preparation may be considered [66]. If three meals per day do not provide an adequate nutritional intake, a smaller and frequent meals are recommended [68-70]. A late-evening snack of 200 kcal improves the nutritional status in patients with cirrhosis and intractable ascites [71-73]. If the patient is actively ill or in a critical state, higher protein (1.5 g/kg/day) and caloric intakes (40 kcal/kg/day) can be considered in conjunction with medical treatment.
While long-term oral or enteral nutrition is thought to be helpful for patients with alcoholic liver cirrhosis, additional studies are required to see the impact of these management. Most studies regarding this issue are limited by small sample sizes and/or insufficient treatment periods [74]. However adequate nutritional therapy reduces complications of alcoholic liver cirrhosis and is not harmful. Currently there are no clear guidelines regarding the supplementation of vitamins or minerals in patients with liver cirrhosis and ascites. However, adequate amounts of vitamin A, thiamine, vitamin B12, folic acid, pyridoxine, vitamin D, and zinc can be considered for supplementation in case of nutritional deficiency [66,75]. Zinc is involved in albumin and BCAA metabolism, and zinc supplements improve ascites and encephalopathy [76,77]. It is important to educate and counsel patients, caregivers, and medical staff about salt intake, diuretics use, and nutrition in the treatment of patients with cirrhosis and ascites. In a study of 77 patients with hepatocellular carcinoma and ascites, active nutritional education improved the prognosis of the patients [11,78].
Sodium intake restriction: The mechanisms responsible for ascites formation in liver cirrhosis include renal functional abnormalities that favor sodium and water retention. The mainstays of treatment include dietary sodium restriction and natriuresis by using oral diuretics [79]. A low-salt diet is considered effective for controlling ascites and shortening hospitalization. Less than 5 g/day of salt intake (sodium: 2 g/day, 88 mmol/day) is recommended. Greater dietary sodium restriction is not recommended because it may worsen the malnutrition that is usually present in these patients [80]. Patients who do not follow a low-salt diet can control ascites by increasing their diuretic dose while allowing an appropriate amount of salt. Body water is passively released by excretion of sodium in the kidney, hence, fluid restriction is not usually necessary for patients with cirrhosis and ascites.
Admission and bed rest: Theoretically, renin-angiotension-aldosteron and sympathetic neverous system activity increase and glomerular filtration and sodium excretion reduce at the time of standing. However, there are no trials to support best rests, and excessive best rest may not only be impractical but may also cause problems such as muscle atrophy [81]. Patients with ascites can be managed in outpatient basis, but hospitalization is recommended in cases complicated by upper gastrointestinal bleeding, hepatic encephalopathy, bacterial infection, hypotension, and liver cancer.
Medications
Diuretics: A low-salt diet alone is often unsuccessful in controlling ascites in patients with cirrhosis. For quicker recovery of symptom and sodium balance, diuretics are used in case of ascites Grade 2 or 3 [82]. Oral administration of diuretics is standard, and intravenous use is not recommended because it can cause kidney damage due to sudden body fluid loss.
Secondary hyperaldosteronism in patients with liver cirrhosis induces reabsorption of sodium and water in the distal renal tubule and collecting tubule, causing hypokalemia. Aldosterone antagonists inhibit this mechanism, and hence are commonly used for controlling ascites in patients with liver cirrhosis. Spironolactone has a long half-life and a slow onset of action. It requires three to four days to achieve a stable concentration. Spironolactone is initiated at a dose of 50–100 mg/day, with a maximum dose of 400 mg/day. Side effects include hyperkalemia, gynecomastia, mastalgia, hyposexuality, and erectile dysfunction [83]. Amiloride has less diuretic effect than spironolactone, but has less anti-androgen effect. Amiloride (10-40 mg/day, 1/10 dose of spironolactone) can be substituted for spironolactone in patients with tender gynecomastia [84].
Loop diuretics act on the Na-K-2Cl receptors in the thick ascending limb of Henle’s loop. Furosemide, a representative loop diuretic, has rapid onset of action. Hypokalemia may occur as a side effect, but hyperkalemia caused by aldosterone antagonists can be corrected. The starting dose is 20–40 mg/day, with a maximum dose of 160 mg/day. Torasemide is characterized by a longer half-life and longer duration of action than furosemide, and is used at a quarter of the dose of furosemide [85].
Aldosterone antagonist is the mainstay of diuretic treatment. Loop diuretics can be used as a combination therapy with aldosterone antagonist, sequentially or initially [86]. Monotherapy with loop diuretics is not recommended. For sequential use, spironolactone monotherapy can be started and furosemide is added in case of insufficient response to spironolactone monotherapy, or in case of hyperkalemia related to spironolactone monotherapy [86]. Initial combination therapy of aldosterone antagonist and loop diuretics can also be considered, using a ratio of 100:40 of spironolactone and furosemide that can maintain adequate serum potassium levels. Combination therapy yielded a faster control of ascites with lower risk of developing hyperkalemia compared to aldosterone monotherapy in case of recurrent ascites [87].
Diuretics should be used as small dose as possible when the ascites is controlled to prevent complications. In cases of hepatic encephalopathy, hyponatremia below 120 mmol/L (despite water restriction), acute kidney injury (AKI), or lack of response in weight with a low-salt diet (<5 g/day), diuretics should be stopped and the patient’s status should be reevaluated [88]. When using diuretics, changes in body weight, vital signs, serum creatinine (sCr), sodium, and potassium should be periodically monitored. If the serum sodium level decreases below 125 mmol/L, diuretics can be carefully reduced or discontinued, and fluid restriction can be considered [80]. Loop diuretics should be reduced or stopped in case of hypokalemia. Aldosterone antagonist should be reduced or stopped in case of hyperkalemia.
Weight control: There is no limit to weight loss per day when peripheral edema is present, however, the patient’s condition should be carefully considered to determine amount of weight loss per day. For patients without edema, a maximum weight loss of 0.5 kg/day is recommended [13,89]. Daily urine sodium excretion can be measured to evaluate the resonse of the diuretics and low salt diet [11,55]. Currently recommended low salt diet (5 g/day) contains sodium 88 mmol/day. About 10 mmol/day of sodium is excreted in non-urinary body fluids such as sweat. Therefore, the excretion of urine sodium should be equal to 78 mmol/day to maintain sodium balance in patients taking low salt diet. For patient not responding to low salt diet and diuretics, it can be considered that low salt intake was not followed by the patient (sodium intake was more than 88 mmol/day) if urinary sodium excretion is over 78 mmol/day. If urinary sodium excretion is under 78 mmol/day, sodium excretion is inadequate and increase in diuretics dose can be considered. Collecting urine and measuring 24 hour sodium is cumbersome to measure it every day, and can be replace with a random urine sodium/potassium ratio (spot urine Na/K ratio) [90]. A spot urine Na/K ratio of more than 1 represents a sodium excretion rate of more than 78 mmol/day, with 90-95% confidence [91]. A spot urine Na/K ratio can be tested regardless of time, as there is no difference in morning or afternoon test results [92].
Branched-chain amino acid supplementation: Long-term oral BCAA supplementation improves nitrogen balance, hepatic encephalopathy, and liver enzyme profiles in patients with hypoalbuminemia [93,94]. A daily supply of 34 g of protein, including BCAA, reduced the number of hospitalizations due to infection, gastrointestinal bleeding, ascites, or hepatic encephalopathy in patients with symptomatic alcoholic cirrhosis [95]. Treatment with BCAA in 204 patients with decompensated cirrhosis for 24 weeks resulted in an increase in albumin and a decrease in ascites and edema [96]. In a randomized comparative study, more than one year of treatment with BCAA significantly reduced the incidence of ascites [97]. A different study demonstrated that administration of BCAA to 21 patients with liver cirrhosis improved albumin levels and increased muscle mass [98]. In another study of patients with liver cirrhosis, high-protein (1.2 g/kg) and high-fiber (30 g fiber) diets with a BCAA preparation increased muscle mass and prevented hepatic coma [99]. In patients with hepatocellular carcinoma (HCC) who underwent liver resection, the use of BCAA was effective in preventing ascites and pleural effusion [100]. In related studies conducted in patients with liver cirrhosis, long-term BCAA therapy improved bilirubin levels, Child-Pugh scores, albumin levels, and survival rates [101,102].
Albumin: Albumin carries loop diuretics to the kidneys [103]. Administration of albumin increased the response to diuretics and reduced hospitalization days [104]. In a meta-analysis, administration of albumin significantly reduced side effects from large-volume paracentesis (LVP) and reduced mortality [105]. In a randomized controlled trial of patients with spontaneous bacterial peritonitis, administration of albumin reduced the incidence of hepatorenal syndrome [106]. In a randomized clinical trial in patients with ascites, the survival rate of patients treated with albumin (25 g/week for one year and then bi-weekly albumin administration) was higher than that of the diuretic alone group [107]. In a recent report by an Italian group, the administration of 6-8 g of albumin per liter of paracentesis was recommended for the prevention of adverse effects after large-volume paracentesis (more than 5 L). To prevent renal damage after treatment of spontaneous bacterial peritonitis, high-risk patients (more than 4 mg/dL of bilirubin or more than 1 mg/dL of sCr) were advised to receive 1.5 g/kg albumin at diagnosis, and 1 g/kg albumin at 3 days [108].
Therapeutic paracentesis: Therapeutic paracentesis refers to draining a large amount of ascites for therapeutic purposes in patients with abdominal wall distension [109]. LVP is safe when 8 g of albumin per 1 liter of ascites is administered. Therapeutic paracentesis is an effective treatment for patients with refractory ascites. It is faster than the use of diuretics alone, and shortens the length of the hospital stay [110].
[Recommendations]
1. Treatment of underlying disease is important in patients with cirrhotic ascites (A1).
2. Supplementation of protein (1.2-1.5 g/kg/day) is recommended in patients with cirrhotic ascites (B1).
3. In patients with cirrhotic ascites, the recommended intake of salt is 5 g/day or less (sodium 2 g/day, 88 mmol/day). Fluid restriction is not necessary if the serum sodium concentration is in the normal range (B1).
4. In the case of peripheral edema, there is no limitation on weight loss/day, but weight loss/day should be decided carefully considering the condition of the patient. In the absence of peripheral edema, weight loss of 0.5 kg/day is recommended (A1).
5. The primary diuretic drug used for patients with cirrhotic ascites is an aldosterone antagonist. Spironolactone is recommended at a starting dosage of 50-100 mg/day, increasing to 400 mg/day (A1). Furosemide, a loop diuretic, can be used in combination to increase the diuretic effect and maintain normal serum potassium levels. Furosemide is recommended at a starting dosage of 20-40 mg/day, increasing to 160 mg/day (A1).
6. When hypokalemia occurs, the loop diuretic should be reduced or stopped. When hyperkalemia develops, the aldosterone antagonist should be reduced or stopped (B1).
7. In cases of severe hyponatremia, acute kidney injury, overt hepatic encephalopathy, or severe muscle spasm, diuretics dose should be reduced or stopped (B1).
8. In the case of therapeutic large-volume paracentesis, 6-8 g of albumin infusion per liter of ascites drained is recommended (A1).
REFRACTORY ASCITES
Definition and diagnosis of refractory ascites
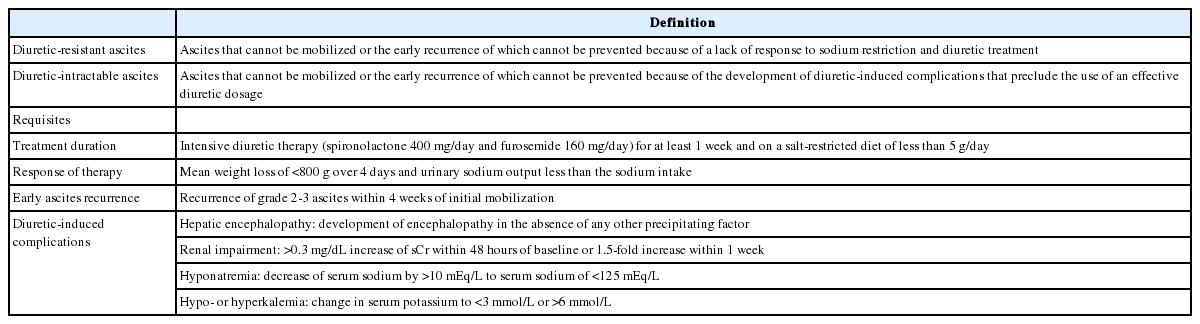
Refractory ascites is defined as fluid overload which 1) fails to respond to a restriction of salt intake and the maximum dose of diuretic treatment (spironolactone at 400 mg/day and furosemide at 160 mg/day), or 2) reappears rapidly after therapeutic paracentesis [111]. Refractory ascites is classified into diuretic-resistant and diuretic-intractable forms (Table 5) [112].
Management of refractory ascites
Large-volume paracentesis
Serial LVP is an effective management strategy for refractory ascites. LVP is not a first-line option for all patients with ascites. It is performed on selected patients who have difficulty eating or breathing due to abdominal distension. After LVP, maintenance therapy should be followed. Compared with diuretic treatment, LVP with intravenous albumin replacement shortens the length of the hospital stay and reduces the risk of hyponatremia, AKI, and hepatic encephalopathy. However, repeated LVP increases the risk of infection and malnutrition related to protein loss [113]. In order to reduce the need for LVP, a salt-restricted diet is recommended.
When diuretic-resistant ascites develops, diuretics treatment is generally discontinued. The European Association for the Study of the Liver recommends discontinuing diuretics when urinary sodium excretion is < 30 mmol/day [13]. As diuretic-resistant ascites is controlled by paracentesis thereafter, the interval and amount of paracentesis reflects a patient’s degree of compliance to a low-salt diet. In Korea, the mean daily sodium intake is 200-300 mmol. However, sodium intake can be reduced to 88 mmol/day or less if a patient maintains a low-salt (up to 5 g/day) diet [114]. In general, patients with refractory ascites excrete less than 20 mmol/day of sodium in the urine. About 10 mmol/day is additionally excreted by insensible loss with body fluids such as sweat. Thus, even if a patient maintains a low-salt diet, more than 60 mmol sodium per day remains in the body. If more than 10 L of paracentesis is needed during a two-week period, the patient is clearly not complying with a low-salt diet.
LVP may shorten the survival of patients due to post-paracentesis circulatory dysfunction [115]. For LVP of more than 5 L, infusion of 6-8 g of intravenous albumin per liter of drained ascites is recommended. Although the incidence of post-paracentesis circulatory dysfunction is relatively low after drainage of <5 L of ascites, colloid replacement (mainly intravenous albumin infusion) can be considered [13,116]. Midodrine [117] or terlipressin [118] can be also used to prevent circulatory dysfunction after LVP.
Medical treatment
In patients with refractory ascites, non-selective beta-blockers (NSBBs) may lower blood pressure and increase the frequency of paracentesis-induced circulatory dysfunction, which may exacerbate renal function. It has been shown that NSBBs shorten the survival of patients with refractory ascites [119]. Thus, the risks and benefits of NSBBs should be carefully weighed in patients with refractory ascites, and consideration must be given to discontinuing NSBBs in patients who are already using them [120]. A NSBBs-induced decrease in the mean arterial pressure is a poor prognostic factor in the decompensated cirrhosis of patients with ascites [121]. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers are not recommended in these patients for the same reason [13].
In addition to standard diuretic treatment, oral midodrine (7.5 mg three times daily) or clonidine (0.1 mg twice daily) can be beneficial in controlling refractory ascites [122]. In particular, additional midodrine on standard diuretic treatment has been shown to increase urine volume, urine sodium, mean arterial pressure, and survival in patients with refractory ascites [123]. Additional use of clonidine has yielded diverse responses associated with the α2-adrenoreceptor polymorphism [124]. Vaptan, a selective V2 receptor blocker, was not more effective in controlling refractory ascites than diuretics treatment; rather, it increased the risk of mortality when used in combination with diuretics [125].
In patients with a poor response to medical treatment, including those discontinuing NSBBs and additional midodrine or clonidine treatment, other options might be applied. These include serial LVP, liver transplantation, TIPS, and peritoneovenous shunt, and other experimental options.
Transjugular intrahepatic portosystemic shunt
In patients with refractory ascites, TIPS can reduce the risk of ascites recurrence and improve the survival rate compared with serial LVP [126]. However, TIPS is an expensive and invasive procedure. Moreover, hepatic encephalopathy occurs in 30-50% of patients who receive TIPS. There is no significant difference in the hepatic encephalopathy incidence between TIPS and serial LVP [127]. However, encephalopathy is more severe in patients with TIPS patients [128]. This could worsen the quality of life and should be acknowledged [129]. It takes time to eliminate ascites after TIPS, and most patients require maintenance of diuretics and salt restriction. Since diuretic resistance may be improved by TIPS, titrating the diuretic dose may be required.
Polytetrafluoroethylene-covered stents reduce the rate of stent obstruction. In a recent study, a polytetrafluoroethylene-covered stent with a 10 mm-diameter was more effective in controlling refractory ascites (without increasing the encephalopathy risk) than a stent with a diameter of 8 mm [130]. Patients received TIPS have a lower risk of liver transplantation than patients who received serial LVP during the first year of follow-up [131,132]. Those who undergo TIPS before liver transplantation show a lower mortality rate than those who do not receive TIPS (adjusted hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.90-0.99) [133]. A retrospective study has suggested that surgical shunts are more effective for refractory ascites than TIPS [134], but prospective comparison studies are needed. Cirrhotic patients usually have a high left-ventricular ejection fraction of >70-75% due to pathophysiological changes. TIPS can induce diastolic heart failure in patients with diastolic dysfunction and an ejection fraction of 50-60%, which may consequently shorten the expected residual survival [135,136]. In patients with renal impairment, and especially in patients on dialysis, the effect of TIPS may be attenuated [137].
Liver transplantation
Patients with refractory ascites often require liver transplantation because 21% of patients die within six months, and the median survival is less than one year [138,139]. Patients with refractory ascites tend to have a poor prognosis, even if the MELD score is relatively low (below 18). Hyponatremia, which is common in patients with refractory ascites, is also associated with a poor prognosis [140]. For these reasons, additional prognosis prediction models (e.g. MELD-Na) have been introduced [141].
Other experimental options
In a retrospective study, administration of albumin (50 g per week) reduced the body weight of patients with refractory ascites who did not meet the indications for TIPS, but further prospective studies are warranted [142]. A randomized pilot study showed that patients who used clonidine along with spironolactone had shorter hospital stays than patients who underwent serial LVP with intravenous albumin infusion [143].
Although peritoneovenous shunt has been performed for refractory ascites since the 1970s, it causes many procedure-related complications and offers no benefit over medical treatment in terms of survival [144]. Therefore, peritoneovenous shunt should be considered only for patients who are not candidates for liver transplantation, and who have poor access to serial LVP. It can be also applied to patients with abdominal wounds, which limit serial LVP. A medical device that drains ascites into the urinary bladder has been developed, and recent clinical trials demonstrated that the device improved the quality of life for patients by reducing the requirement for serial LVP. However, side effects, such as AKI were also reported [145]. Indwelling catheters and ports may be useful in malignant ascites, but their safety and efficacy have not been clearly demonstrated in cirrhosis-induced ascites.
Cell-free and concentrated ascites reinfusion therapy (CART) during LVP may be considered in Asian patients who have low body mass. During CART, concentrated ascites fluid is reinfused after the removal of cells. CART appears to be as effective as albumin infusion and may reduce the albumin consumption [146,147].
[Recommendations]
1. Liver transplantation is recommended in patients with refractory ascites (A1).
2. Patients with refractory ascites should maintain a low-salt diet and control their ascites with serial large-volume paracentesis (A1).
3. For large-volume paracentesis in patients with refractory ascites, 6-8 g of albumin infusion per liter of ascites drain is recommended (A1).
4. A transjugular intrahepatic portosystemic shunt can be performed for the management of refractory ascites (A2).
5. Beta-blockers should be used with caution, and careful monitoring of blood pressure and renal function is required for patients with refractory ascites (B1).
HYPONATREMIA
Hyponatremia is a commonly observed complication related to hypoalbuminemia and portal hypertension in patients with advanced liver cirrhosis. Generally, hyponatremia is defined as a serum sodium concentration less than 135 mmol/L. Hyponatremia in patients with liver cirrhosis is mostly dilutional hyponatremia, and is defined at a serum sodium concentration below 130 mmol/L [13,81,148,149]. This is because the risk of complications increases significantly in patients with hyponatremia below 130 mmol/L and liver cirrhosis accompanied by ascites. Complications include spontaneous bacterial peritonitis (odd ratio [OR], 3.40; 95% CI, 2.35-4.92), hepatorenal syndrome (OR, 3.45; 95% CI, 2.04-5.82), and hepatic encephalopathy (OR, 2.36; 95% CI, 1.41-3.93). More evidence is needed to establish the starting point of treatment for hyponatremia [150].
Pathophysiology
Hyponatremia in liver cirrhosis is caused by systemic vasodilatation due to a deterioration of portal hypertension, and by a decreased effective plasma volume [151]. This causes a decrease in systemic vascular resistance, a decrease in mean arterial blood pressure, and an increase in cardiac output. Ultimately, it induces hyperdynamic circulation [150,152]. In particular, nitric oxide, glucagon, vasoactive intestinal peptide, substance P, platelet activating factors, prostaglandins, and prostacyclins accumulate and contribute to splanchnic arterial vasodilation [153-155].
Systemic vasodilation and decreased effective plasma volume stimulate the body to maintain effective plasma volume through the renin-angiotensin-aldosterone system. This results in excessive reabsorption of sodium and water. Eventually, lower extremity edema and ascites are clinically observed [156]. Hyponatremia is particularly severe in patients with decompensated liver cirrhosis because the regulation of antidiuretic hormone in accordance with body water is inadequate [157]. Increased arterial natriuretic peptide, decreased prostaglandin E2, and decreased degradation of antidiuretic hormone also aggravate hyponatremia [156].
Treatment of hyponatremia
Treatment according to the cause of hyponatremia
The first step in the treatment of hyponatremia is to distinguish the type of hyponatremia. Fluid resuscitation is needed for hypovolemic hyponatremia. In patients with liver cirrhosis, hypovolemic hyponatremia caused by excessive diuretic use is common. Withdrawal of diuretics or correction of other possible cause of dehydration should be considered. In these patients, hypertonic sodium chloride administration can be considered. However, this requires attention because an excessive correction of the serum sodium concentration can cause many side effects or complications. In particular, a correction of more than 9 mmol/L within 24 hours is associated with central pontine myelinolysis or seizures. Frequent monitoring is necessary when correcting the serum sodium concentration [158].
In cases of hypervolemic hyponatremia, discontinuation of intravenous fluid therapy and free water restriction should be considered. If the serum sodium concentration is below 120-125 mmol/L and neurologic symptoms are present, fluid restriction (1-1.5 L/day) should be considered. The effect of restricting fluid intake on the serum sodium concentration is unclear, and prospective studies are lacking. However, an indirect analysis of patients who underwent fluid restriction as a control group in clinical trials showed that, at least, fluid restriction could prevent deterioration of serum sodium level below a certain level [159,160]. Plasma expander such as albumin infusion has been tried, and was reported to be effective in hyponatremia. However, the number of patients was very small [161]. The administration of hypertonic sodium chloride allows a temporary elevation in the serum sodium concentration and symptom relief after administration, but this treatment requires close attention because edema and ascites can be worsened.
Vaptan
In terms of the pathophysiology of hypervolemic hyponatremia, an ideal therapy should encourage the excretion of solute-free water to prevent losing electrolytes through urination. The recently developed vaptan drugs selectively inhibit the V2 receptor of vasopressin, an antidiuretic hormone of the prinical cell in the collecting duct of the urinary tract. Vaptans selectively suppress water reabsorption, thereby enhancing urinary excretion. Without vaptans, systemic vasodilation and decreased effective plasma volume increase vasopressin and water reabsorption in patients with hypervolemic hyponatremia. In particular, vaptans are effective in patients with the syndrome of inappropriate antidiuretic hormone secretion (SIADH), heart failure, and liver cirrhosis. Clinical studies have shown that vaptans can enhance urinary excretion without affecting renal function, urinary sodium excretion, cardiovascular function, or the renin-angiotensin-aldosterone system. Several vaptans are currently available in clinical practice, and can be used intravenously (conivaptan) or orally (lixivaptan, satavaptan, and tolvaptan).
Conivaptan is a dual arginine vasopressin antagonist with affinity for the human V1A and V2 receptors [162-164]. It is an intravenous drug approved by the Food and Drug Administration since 2005 for the treatment of euvolemic and hypervolemic hyponatremia [165]. It binds to human plasma proteins when administered by injection. It is metabolized by CYP3A (an intrahepatic enzyme), and 83% of the metabolites are excreted through the stool [166]. It is used for short periods of 2-4 days for the improvement of euvolemic or hypervolemic hyponatremia. Frequent adverse events reported in clinical studies included phlebitis and hypersensitivity at the injection site, observed in 70% of patients. In addition, minor headache, thirst, constipation, and nausea were reported [165]. Serious adverse events, including excessive hypotension and a rapid increase in the serum sodium concentration, occurred in 10% of patients [167]. Hypokalemia occurred in 20% of patients. Arrhythmia and rhabdomyolysis due to hypokalemia occurred in some patients with heart failure [165]. Adverse effects such as deterioration of liver function or hepatic failure have not been reported, but 50% of the dose is recommended in patients with uncompensated liver cirrhosis because metabolism of the drug is approximately 60% slower [168].
Lixivaptan is a very potent oral drug that functions as a non-peptide V2 receptor antagonist. In 60 patients with liver cirrhosis accompanied by hyponatremia, serum sodium levels were normalized in 27% and 50% of patients dosed with 100 mg and 200 mg of lixivaptan, respectively [159]. However, both treatment groups had side effects including severe dehydration and hypotension. In a placebo-controlled study performed with three different doses (25, 125, 250 mg) in patients with liver cirrhosis accompanied by ascites, there was a dose-dependent increase in urine volume, reduction in body weight, and rise in the serum sodium concentration. However, 12 of 32 clinical study participants dropped out owing to dehydration, thirst, and hypotension caused by an excessive increase in urine volume during the study period (8 days). The drugs were discontinued because of the rapid increase in the serum sodium concentration [160]. As side effects were frequent and severe in patients with liver cirrhosis, trials have since been conducted mainly on patients with heart failure.
There was improvement of hyponatremia in patients with SIADH who were treated with 25-50 mg/day of satavaptan for approximately 12 months, suggesting the possibility of long-term hyponatremia correction [169]. When satavaptan was administered at 5-25 mg/day for 14 days, there was improvement in hyponatremia (average 4.5-6.6 mmol/L increase in the serum sodium concentration, compared with levels before medication) and ascites (average 1.5-1.6 kg body weight reduction) in patients with liver cirrhosis, relative to a placebo group. Satavaptan reduced the recurrence of ascites and increased the diuretic effect, regardless of hyponatremia [170,171]. In a large-scale, phase III study comparing satavaptan with placebo in 1,200 patients with liver cirrhosis accompanied by ascites, hyponatremia was improved in only eight days with 5-10 mg/day (OR, 2.91; 95% Cl, 1.46-5.78). Furthermore, no significant differences in complications or survival rates were observed (compared to a placebo group) when satavaptan was used for 52 weeks. In one subgroup analysis of patients with reduced baseline liver function, there were increased side effects and higher mortality rates due to complications (HR, 1.47; 95% CI, 1.01-2.15). Therefore, caution is needed when administering satavaptan for long-term periods [125].
Tolvaptan, an oral medication, significantly improved weight gain and hyponatremia (compared with a placebo group) in a study of patients with congestive heart failure [172]. When 120 patients with liver cirrhosis received 15-60 mg of tolvaptan for 30 days, there was a significant improvement in the serum sodium concentration by the fourth day of treatment. This was well maintained until the 30th day (the end-point of treatment), and gradually dropped to the level of the control group after termination of treatment. There were no serious side effects, although dry mouth and thirst were reported [173]. As an extension of the above study [111], patients who received more than 15 mg of tolvaptan for more than two years in order to verify its long-term stability and efficacy. Among them, six patients dropped out owing to thirst, fatigue, or polyuria. There were no significant side effects over the long-term period except in one patient, who stopped medication because of hypernatremia [174]. However, 4.4% of the tolvaptan treatment group (vs. 1.0% of the placebo group) had elevated alanine aminotransferase (ALT) levels. In fact, their ALT levels were more than three times the normal upper limit defined in a phase III study of tolvaptan in patients with autosomal dominant polycystic kidney disease. Therefore, the U.S. Food and Drug Administration has limited tolvaptan treatment in patients with liver cirrhosis or impaired liver function. The European Medicines Agency approved the use of tolvaptan in 2015, but has recommended monthly liver function tests in patients with autosomal dominant polycystic kidney disease [174-178]. In China and Japan, low doses of tolvaptan (7.5-15 mg/day) have been approved to control ascites, with a warning that liver dysfunction may occur [179].
Prognosis of hyponatremia
Hyponatremia is associated with mortality in patients with liver cirrhosis and ascites. The risk of refractory ascites increases and frequent therapeutic paracentesis is required when the serum sodium concentration drops below 135 mmol/L [150,180]. In cases where the serum sodium concentration drops below 130 mmol/L, quality of life markedly decreases due to dietary regulations for ascites control and diminished cognitive function [181,182]. Patients with hyponatremia are frequently exposed to spontaneous bacterial peritonitis, higher risk of hepatorenal syndrome and death, showing poorer prognosis [182]. The MELD-Na score, which adds the sodium level to the calculation of MELD score, is used to determine the prognosis of end-stage cirrhosis. The prognosis is poorer when accompanied by hyponatremia. MELD-Na score is used to prioritize liver transplant candidate in the United States [183]. Hyponatremia is also known to affect the overall survival after transplantation, and serious neurological complications can occur if hyponatremia is corrected rapidly after transplantation [184].
[Recommendations]
1. When the serum sodium concentration decreases to less than 130 mmol/L in patients with liver cirrhosis and ascites, most are dilutional hyponatremia. Hyponatremia requires special attention as it is associated with a poor prognosis and multiple complications (A1).
-
2. Fluid intake can be restricted to 1.0-1.5 L/day in cases of dilutional hyponatremia when the serum sodium concentration falls below 120-125 mmol/L (B1).
Administration of a plasma expander, such as albumin, may be considered for the treatment of hyponatremia (B2).
SPONTANEOUS BACTERIAL PERITONITIS
Definition and diagnostic criteria
Definition
Spontaneous bacterial peritonitis (SBP) is bacterial infection of ascites, without an evident intra-abdominal, surgically treatable source of infection. SBP occurs in 20-30% of patients with cirrhotic ascites [12,185], and its mortality rate is approximately 20% [186].
Diagnosis
An abdominal paracentesis should be performed and ascites fluid should be analyzed in patients with signs of peritonitis (abdominal pain, vomiting, ileus, etc.) or other signs of infection. This also applies to patients with unexplained worsening liver and/or kidney function, or hepatic encephalopathy [13]. SBP can be diagnosed when the ascitic polymorphonuclear leukocyte (PMN) count ≥ 250/mm3, without an evident intra-abdominal infection. If there are red blood cells (RBCs) in the ascites, the PMN count is adjusted by subtracting 1 PMN per 250 RBCs/mm3 [187]. Ascitic fluid for culture should be taken before empirical antibiotics administration. Inoculation of ascitic fluid into blood culture bottles at the bedside is recommended because of the higher growth rate observed (approximately 80%) compared to conventional culture methods (approximately 50%) [188]. Approximately 40% of patients who have an ascitic PMN count ≥250/mm3 are culture-negative (even with the appropriate culture tests), a condition known as culture-negative neutrocytic ascites [187]. Because these patients show a clinical course similar to patients with culture-positive ascitic fluid, empirical antibiotic therapy is recommended [189]. In some patients, a single strain of bacteria is cultured in the ascitic fluid, but the ascitic PMN count <250/mm3 (a condition known as monomicrobial non-neutrocytic bacterascites). These results indicate the colonization of bacteria in the ascites, and asymptomatic patients need no treatment because most of them resolve the colonization without antibiotics [190,191]. In one prospective study, many patients with signs or symptoms of infection, but an ascitic PMN count <250/mm3, progressed to SBP [191]. Patients with signs or symptoms of infection (such as fever or abdominal pain), including patients with unexplained complications (such as renal impairment or hepatic encephalopathy), should therefore receive empirical antibiotics while awaiting the results of culture, even if the ascitic PMN count <250/mm3. It may be possible to diagnose SBP more quickly using the reagent strip test. However, this test is not recommended due to its low sensitivity and high false-negative rate [192-194].
Secondary bacterial peritonitis
Approximately 5% of patients develop secondary bacterial peritonitis caused by intestinal perforation or abscess [195]. Secondary bacterial peritonitis has a high mortality rate (50-80%) [48,196], and surgical treatment should be considered. However, it is important to differentiate between secondary bacterial peritonitis and spontaneous bacterial peritonitis because unnecessary laparotomy in cirrhotic patients increases the mortality rate [197]. Secondary bacterial peritonitis may be suspected in the following cases: 1) the PMN count increases to >1,000/mm3; 2) multiple organisms are seen by Gram stain or in culture using the ascitic fluid; 3) the ascitic total protein concentration ≥1 g/dL; 4) the LDH level in the ascites fluid is above the normal upper limit of LDH in the serum; 5) the ascitic glucose concentration ≤50 mg/dL; and 6) the ascitic PMN count does not drop after 48 hours of antibiotic treatment [48]. Elevated levels of ascitic fluid CEA (>5 ng/mL) or alkaline phosphatase (>240 U/L) are helpful in the diagnosis of secondary bacterial peritonitis caused by intestinal perforation [49]. Clinical manifestations, course of treatment, ascitic glucose levels, and LDH levels may be helpful, however appropriate imaging techniques (such as abdominal computed tomography) are necessary if secondary peritonitis is suspected.
[Recommendations]
1. If spontaneous bacterial peritonitis is suspected and the polymorphonuclear leukocyte count is greater than 250/mm3, the patient should be diagnosed as spontaneous bacterial peritonitis (regardless of the ascitic fluid culture result) and empirical antibiotic therapy should be started (A1).
2. Even if the polymorphonuclear leukocyte count is less than 250/mm3, when symptoms or signs of infection are present (e.g. body temperature > 37.8°C, abdominal pain or tenderness), empirical antibiotic administration is recommended until culture results become available (B1).
3. If secondary bacterial peritonitis is suspected, imaging tests such as abdominal computed tomography should be performed (A1). Tests for ascitic total protein, lactate dehydrogenase, glucose, Gram stain, carcinoembryonic antigen, and alkaline phosphatase help differentiate secondary bacterial peritonitis from spontaneous bacterial peritonitis (B1).
Treatment
Community-acquired spontaneous bacterial peritonitis
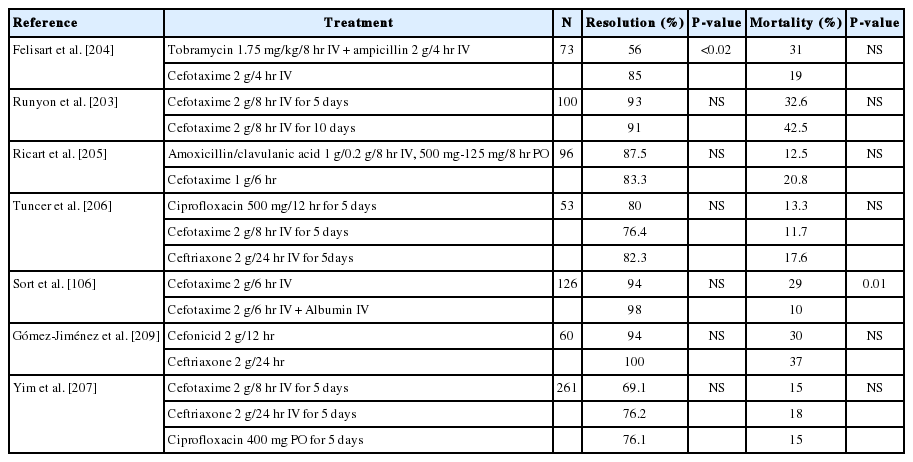
Patients who are suspected of ascitic fluid infection should begin empirical antibiotic therapy before culture and antibiotic susceptibility test results are avialable. The most commonly identified bacteria in culture are Escherichia coli, Klebsiella pneumoniae, and Streptococcus (Table 6) [198-202]. Third-generation cephalosporin antibiotics are recommended as they are effective for most causative bacterial pathogens, including these strains. Cefotaxime is the most studied third-generation cephalosporins. Intravenous cefotaxime treatment in patients with SBP delivers a high concentration of drug to the ascites [203], and yields a high resolution rate of 69-98% [203-207]. In one study, a 5-day treatment group and a 10-day treatment group showed similar therapeutic effects [203]. Intravenous ceftriaxone treatment showed a 73-100% resolution rate, similar to cefotaxime treatment (Table 7) [206,208-210]. Therefore, in patients suspected of SBP, cefotaxime at a dose of 2 g every 6-8 hours, or ceftriaxone at a dose of 1 g every 12-24 hours, are recommended by intravenous injection. The standard treatment duration is 5 to 10 days. However, the treatment duration should vary according to the symptoms and/or results of antimicrobial susceptibility testing. Similarly, antibiotics should be replaced in accordance with the susceptibility results of bacteria cultured from ascites or blood.
Treatment with amoxicillin-clavulanic acid shows similar SBP resolution rates to cefotaxime, and treatment with ciprofloxacin shows similar survival rates to cefotaxime [205,211]. Oral treatment with ofloxacin shows similar therapeutic efficacy to cefotaxime in patients without complications (such as gastrointestinal bleeding, renal dysfunction, hepatic encephalopathy, ileus, and shock) [212]. However, caution is needed because causative oraganims isolated in community-acquired SBP is increasingly resistant to quinolone [198]. Recently, a university hospital in Korea reported that E.coli resistance to quinolone was as high as 31.7% [213]. The risk of quinolone resistance is increased in patients who have previously recovered from SBP and in those who have been exposed to quinolone [213-218]. In patients with these risk factors, the choice of antibiotics should be made taking into account the possibility of infection by quinolone-resistant strains.
Hospital-acquired spontaneous bacterial peritonitis
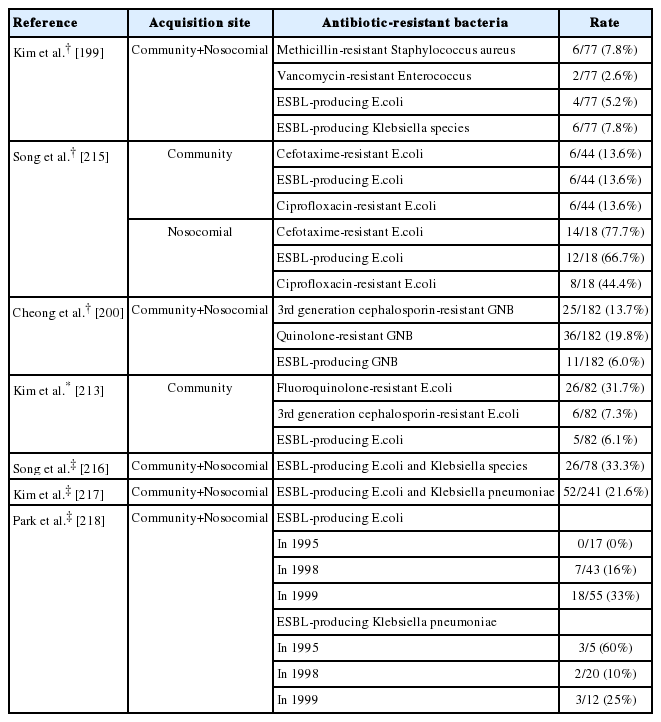
Hospital-acquired SBP is defined as occurring after more than 48-72 hours of hospitalization [219,220]. Hospital-acquired SBP is at risk of treatment failure when third-generation cephalosporins, the first choice of empirical treatment for community-acquired SBP, are used [200,214,215,220,221]. According to various Korean studies, extended-spectrum beta-lactamase (ESBL)-producing bacterial strains account for 5-30% of all SBP cases [200,215-217]. More specifically, ESBL-producing bacteria account for 13-20% of communityacquired SBP cases, but 46-66% of hospital-acquired SBP cases [215,217]. The frequency of ESBL-producing bacteria has also been increasing within the same hospital [198,216,217]. Cases of SBP caused by multidrug-resistant gram-positive bacteria (such as Enterococcus or methicillin-resistant Staphylococcus aureus) have also increased (Table 8) [199,200]. Hospital-acquired SBP has a higher mortality rate than community-acquired SBP, due to increased resistance to third-generation cephalosporins, infection by Gram positive bacteria and multidrug-resistant strains [200,216]. In a randomized, controlled study of patients with hospital-acquired SBP, meropenem-daptomycin treatment was more effective than ceftazidime [219]. An empirical selection of antibiotics should be based on the severity of the infection, the risk factors for multidrug-resistant infection, and local epidemiology [222,223]. Risk factors for multidrug-resistant bacterial infection include hospital-acquired infection, long-term use of prophylactic antibiotics, recent use of beta-lactam antibiotics, and recent history of hospitalization [220,224]. Carbapenem treatment (with or without glycopeptides) is often considered in patients with severe infection or with risk factors for multidrug-resistant bacterial infection. With this treatment, it is necessary to re-evaluate and consider de-escalation after 48-72 hours in order to reduce the chance of developing antibiotics resistance [222]. As a general rule, it is necessary to select empirical antibiotics based on local epidemiology by regularly monitoring of commonly isolated organism and their resistance profiles at each institution.
Follow-up paracentesis
Because most SBP shows a good response to empirical antibiotics, routine follow-up paracentesis to evaluate the treatment response is not needed in patients with SBP. Follow-up paracentesis can be helpful if there are no symptom improvements after treatment, or if secondary bacterial peritonitis is suspected. If the PMN count in the ascitic fluid does not decrease by more than 25% after 2 days of empirical antibiotics [187], this should be considered as treatment failure. For these patients, drugs targeting bacteria that cannot be treated with cephalosporins such as ESBL-producing bacteria, MRSA, enterococcus, and pseudomonas should be considered.
Other treatments
Albumin: Approximately 30-40% of patients with SBP develop renal dysfunction [225,226], and when renal dysfunction develops, the risk of mortality is very high [227]. In patients with SBP, cefotaxime treatment with albumin infusion (1.5 g/kg at the time of diagnosis and 1.0 g/kg at 3 days) decreased the incidence of hepatorenal syndrome (HRS) (10% vs. 33%) and mortality (10% vs. 29%) compared to cefotaxime alone [106]. The incidence of HRS is low (<8%) in patients with serum bilirubin <4 mg/dL and sCr <1.0 mg/dL on diagnosis of SBP [106]. On the other hand, patients with serum bilirubin >4 mg/dL or sCr >1.0 mg/dL are at high risk (58%) for renal dysfunction, and albumin treatment is helpful in these patients [228]. Recently, there was a report that low doses of albumin (1.0 g/kg at the time of diagnosis and 0.5 g/kg at 3 days) are effective in preventing renal dysfunction, but further studies are needed [229].
Non-selective beta-blockers and diuretics: Non-selective beta-blockers (NSBBs) are known to inhibit the development of SBP by reducing intestinal transit time, inhibiting intestinal bacterial overgrowth, and reducing intestinal bacterial translocation [230]. A meta-analysis of patients using NSBBs for the prevention of variceal bleeding showed that NSBBs reduced the incidence of SBP by 12.1% (OR, 0.428; 95% CI, 0.26-0.70) [231]. A retrospective study showed that the use of NSBBs in patients with SBP reduced the risk of transplant-free survival (HR, 1.58; P=0.014) by increasing the risk of HRS and AKI [232]. Another study showed that high-dose NSBBs decreased survival (adjusted HR, 1.30; P=0.059), while low-dose NSBBs increased survival (adjusted HR, 0.34; P=0.03) [233]. Therefore, the role of NSBBs in patients with SBP is still unclear, and clinicians should discontinue or adjust the dose considering the benefits and risks of its use.
The use of diuretics in cirrhotic patients with ascites increases total protein and complement levels in the ascitic fluid. Diuretics also increase opsonic activity in the ascites, thus inhibiting the development of SBP [234]. In one study of patients with SBP, those with a response to diuretics showed elevated total protein and opsonic activity in ascites [235]. However, AKI is common in patients with SBP. Therefore, renal function monitoring is needed, and the dose of diuretics should be reduced or discontinued according to changes in renal function.
Prophylaxis
Primary prophylaxis
Bacterial infections, including SBP, occur in 35-66% of liver cirrhosis patients with gastrointestinal bleeding within 1-2 weeks of admission [236]. In these patients, infection increases treatment failure, re-bleeding, and mortality [237,238]. A meta-analysis of previous studies showed that prophylactic antibiotic therapy in patients with liver cirrhosis with gastrointestinal bleeding reduced severe bacterial infections, re-bleeding, and mortality [236,239]. Administration of oral norfloxacin (400 mg twice for 1 week) is effective in preventing infection in patients with liver cirrhosis accompanied by gastrointestinal bleeding [240]. However, in patients with gastrointestinal bleeding accompanied by severe hepatic dysfunction (two or more factors: ascites, severe malnutrition, bilirubin >3 mg/dL, or hepatic encephalopathy), prophylaxis with ceftriaxone (1 g/day for 1 week) was more effective than oral norfloxacin [241].
Among patients with ascites, those with low protein concentrations in the ascites have a high risk of developing SBP [242-244]. In a double-blind, randomized, controlled study in patients with an ascitic protein concentration <1.5 g/dL, norfloxacin (400 mg/day for 6 months) reduced infections by Gram-negative bacteria, but did not lower the incidence and mortality of SBP [245]. In another double-blind, placebo-controlled study in patients with an ascitic protein concentration <1.5 g/dL, ciprofloxacin (500 mg/day for 12 months) reduced the incidence of SBP from 14% to 4% (albeit with limited statistical significance, P=0.074), and increased the 1-year survival from 66% to 88% (P=0.04) [246]. Thus, the efficacy of prophylactic antibiotics in preventing SBP and reducing mortality is unclear for patients with an ascitic protein concentration <1.5 g/dL. However, prophylactic administration of norfloxacin for 1 year in patients with an ascitic protein concentration <1.5 g/dL accompanied by hepatic dysfunction (Child-Pugh score ≥9 and bilirubin ≥3 mg/dL), renal insufficiency (sCr ≥1.2 mg/dL or blood urea nitrogen ≥25 mg/dL), or hyponatremia (Na <130 mmol/L) reduced the cumulative incidence of SBP from 61% to 7%, decreased the incidence of HRS from 41% to 28%, and reduced mortality from 94% to 62% within 1 year [247]. Therefore, prophylactic administration of norfloxacin (400 mg/day) may be helpful in patients with an ascitic protein concentration <1.5 g/dL, especially when hepatic dysfunction, renal insufficiency, and hyponatremia are present. However, the long-term use of prophylactic antibiotics may increase the likelihood of infection by quinolone-resistant strains or multidrug-resistant strains [213,220].
In a retrospective study using rifaximin as a primary prophylaxis in patients with no history of SBP, its use reduced the incidence of SBP (adjusted HR, 0.28; P=0.007) [248]. A prospective case-controlled study also showed that rifaximin reduces the incidence of SBP (4.5% vs. 46%, P=0.027) [249]. A retrospective study of rifaximin for the treatment of hepatic encephalopathy in Korea showed that its use reduced the incidence of SBP (P<0.001) [250]. However, there was no difference in the incidence of SBP between the rifaximin-treated group and the non-treated group (22% vs. 30%) in another study [251]. Therefore, the use of rifaximin as a primary prophylaxis to prevent SBP requires further studies.
Secondary prophylaxis
Patients recovered from SBP have a recurrence rate of SBP, about 70% within 1 year [252]. After recovery from SBP, norfloxacin (400 mg/day) decreases the recurrence rate from 68% to 20%, and decreases recurrence by Gram-negative bacteria from 60% to 3% [253]. Norfloxacin at 400 mg/day yields a lower tendency of recurrence rate than rufloxacin at 400 mg/week (26% vs. 36%, P=0.16), which was due to a lower rate of recurrence by the Enterobacteriaceae with norfloxacin treatment (0% vs. 22%, P=0.01) [254]. In a prospective study using trimethoprim-sulfamethoxazole (160-800 mg) and norfloxacin for secondary prevention, the recurrence rate of SBP did not differ between the trimethoprim-sulfamethoxazole group and the norfloxacin group (10.0% vs. 9.1%, P=0.50) [255]. However, further studies are necessary as study sample are relatively small. In a randomized, controlled trial comparing rifaximin with norfloxacin, the 6-month cumulative recurrence rate (3.9% vs. 14.1%) and mortality rate (13.7% vs. 24.4%) were lower for rifaximin (1,200 mg/day) than for norfloxacin (400 mg/day) [256].
[Recommendations]
1. Third-generation cephalosporins, such as cefotaxime or ceftriaxone, are recommended as empirical antibiotics for community-acquired spontaneous bacterial peritonitis (A1).
2. In patients with hospital-acquired spontaneous bacterial peritonitis, history of prolonged use of prophylactic antibiotics, recent use of beta-lactam antibiotics, or recent hospitalization, the risk of infection by multidrug-resistant bacteria should be considered when choosing antibiotics (B1).
3. In patients with spontaneous bacterial peritonitis, the albumin infusion reduces the risk of hepatorenal syndrome (A1).
4. In patients with liver cirrhosis accompanied by gastrointestinal bleeding, intravenous ceftriaxone (1 g/day) is recommended (A1). Oral norfloxacin (400 mg twice, i.e. 800 mg/day) can be considered if hepatic dysfunction is not severe (A2).
5. In patients with ascitic protein level of <1.5 g/dL, norfloxacin (400 mg/day) can be considered for primary prevention of spontaneous bacterial peritonitis if severe hepatic dysfunction, renal insufficiency, or hyponatremia co-exist (A2).
6. Patients recovered from spontaneous bacterial peritonitis have a high risk of recurrence, and norfloxacin (400 mg/day) can be considered to prevent recurrence of SBP (A2). Rifaximin (1,100-1,200 mg/day) can be used as an alternative to norfloxacin as a secondary prophylactic agent (B1).
ACUTE KIDNEY INJURY AND HEPATORENAL SYNDROME
Definition, diagnosis, and prevention
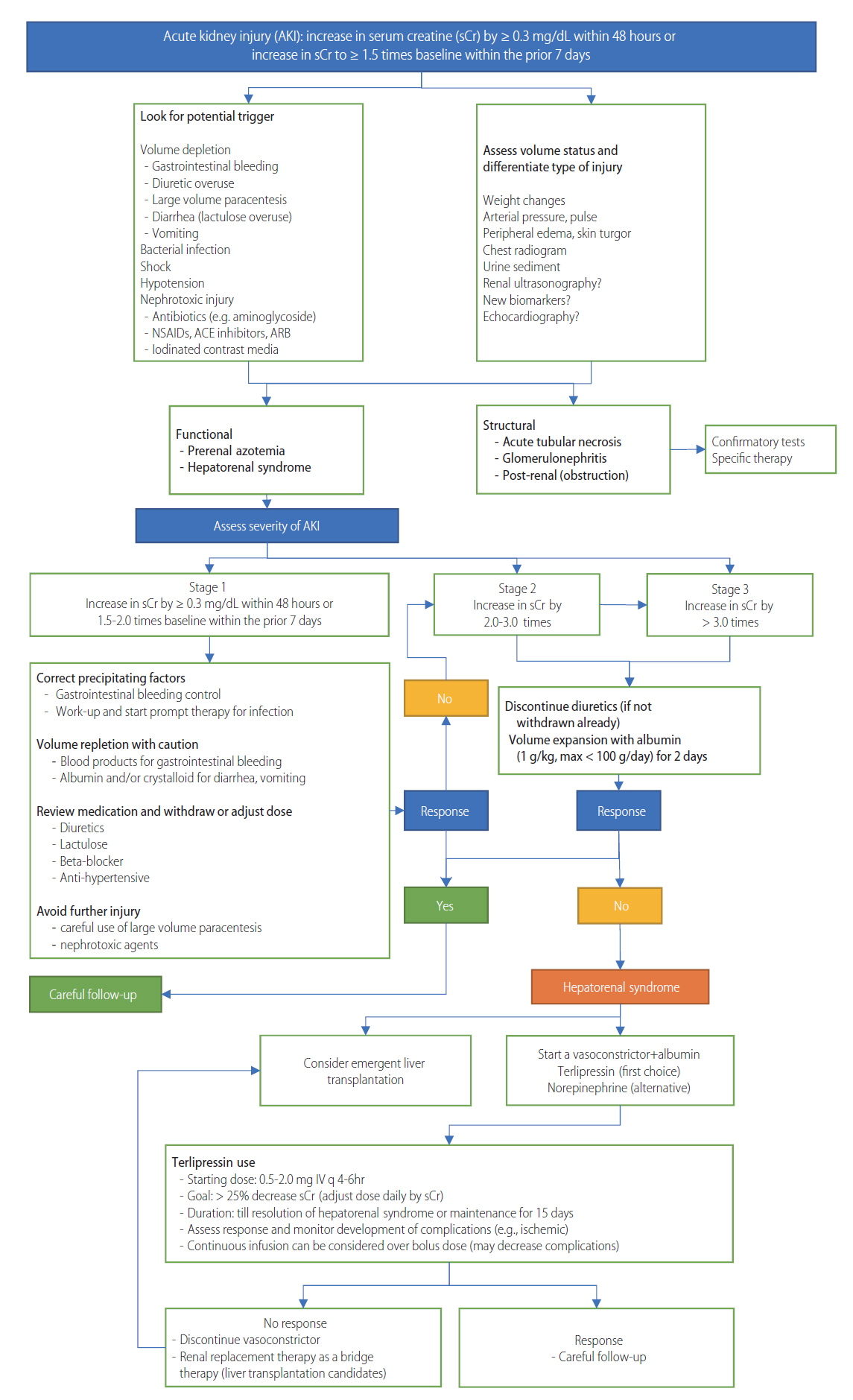
Acute kidney injury (AKI) is common in patients with liver cirrhosis, occurring in 13-20% of hospitalized patients with decompensated cirrhosis [257,258]. It is significantly associated with a patient’s prognosis [227,259,260]. The development and progression of AKI is an independent predictive factor for mortality in these patients [260,261]. If AKI develops (even with later improvements), renal function progressively declines, and patients have a worse prognosis than those without a history of AKI [262]. In patients without appropriate treatment, or without improvement after the initial treatment, AKI often progress to HRS. HRS is associated with significant morbidity and mortality [263]. Although liver transplantation is considered the only definitive treatment for HRS, pre-transplant renal function can affect post-transplant morbidity and mortality [264]. The three-year survival rate after liver transplantation is about 80% in patients without prior HRS, and is about 60% in patients with prior HRS. Patients with prior HRS before liver transplantation have a higher incidence of renal replacement treatment [265]. Therefore, it is necessary to improve the renal function before transplantation.
AKI in patients with liver cirrhosis can be classified into two groups: functional injury and structural injury. In about 70% of cases with cirrhosis, AKI is a functional injury caused by pre-renal failure due to gastrointestinal hemorrhage, bacterial infection, hypovolemia by overuse of diuretics, LVP, diarrhea by overuse of non-absorbable disaccharide (lactulose or lactitol), or reduced renal blood flow by NSBBs-induced hypotension [266]. The development of functional injury in patients with cirrhosis is caused by altered systemic hemodynamics [267]. Liver cirrhosis and portal hypertension-induced splanchnic and systemic vasodilation lead to a reduction in the effective arterial volume. This activates the renin-angiotensin-aldosteron system and sympathetic nervous system, and induces renal injury [268]. These changes induce sodium and water retention, promote the development of ascites and hyponatremia, and trigger renal impairment by reducing renal blood flow and renal arterial vasoconstriction, which can progress to HRS [226,269,270]. The incidence of HRS is significantly increased in patients with left ventricular diastolic dysfunction [271], or relative adrenal insufficiency [272,273]. In 70% of cases, the functional renal disorder is a pre-renal azotemia which responds to intravascular volume replacement. In the other 30% of cases, the disorder is HRS, which is not responsive to intravascular volume replacement [257]. About 30% of AKI in patients with cirrhosis is structural injury, as in case of hepatitis B- or hepatitis C-associated glomerulonephropathy or acute tubular necrosis. Acute tubular necrosis can be caused by gastrointestinal hemorrhage, overuse of diuretics, LVP-induced hypotension, toxins, antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), or computed tomography contrast agents [266,274]. Post-renal AKI by urinary tract obstruction can lead to the development of AKI in these patients, but the incidence is very low (<1%) [275].
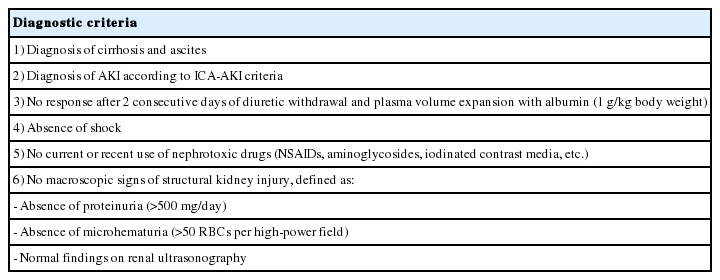
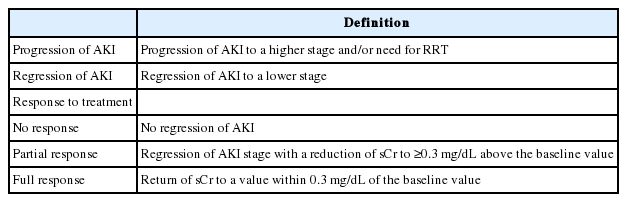
Diagnostic criteria
Acute kidney injury: Traditionally, AKI in cirrhosis has been defined using sCr levels (>50% increase in the sCr level from baseline, or a final value >1.5 mg/dL) [111,276]. However, the sCr level is a poor marker for renal function in patients with cirrhosis. These patients show a reduced production of sCr from significant muscle wasting [277,278], and an increase in the renal tubular secretion of sCr [279]. In addition, elevated bilirubin levels may interfere with sCr measurements [280]. Therefore, sCr-based measurements could overestimate the true renal function, which in turn might delay the diagnosis and initiation of treatment for AKI in these patients [281]. Additionally, the use of a fixed threshold of sCr (1.5 mg/dL) may not represent dynamic changes in renal function, which are needed to distinguish between acute and chronic injury [282].
In 2004, the Acute Dialysis Quality Initiative (ADQI) group proposed a definition and classification system for AKI, known as the RIFLE (Risk, Injury, Failure, Loss of renal function, and End-stage renal disease) criteria. These criteria classify the degree of AKI into three stages according to changes in the sCr level and urine volume (Table 9) [283]. The RIFLE criteria do not use the strict sCr cut-off value of 1.5 mg/dL for the diagnosis of AKI; rather, they define AKI as an increase in the sCr level ≥1.5 × the baseline level within 1 week [283]. Several studies have suggested that the RIFLE criteria are useful for predicting in-hospital mortality in cirrhotic patients admitted to the ICU [284,285]. The Acute Kidney Injury Network (AKIN), a collaborative network consisting of experts from ADQI, nephrology societies, and intensive care medicine societies, proposed a new definition for AKI [286]. There were concerns about small increases in sCr levels which might not affect the RIFLE classification, but could be associated with adverse outcomes [287]. The AKIN criteria broadened the definition of AKI to include an absolute increase in sCr of ≥0.3 mg/dL within 48 hours [286]. The AKIN criteria have been useful for predicting the prognosis of patients with cirrhosis [260,288]. In 2012, the Kidney Disease Improving Global Outcomes (KDIGO) Foundation proposed the following definition for AKI: an increase in sCr levels of ≥0.3 mg/dL within 48 hours, or ≥50% from baseline within 7 days, or a decrease in urine volume <0.5 mL/kg/h within 6 hours [289]. In a study involving 242 cirrhotic patients hospitalized in the intensive care unit, the KDIGO criteria were more useful in predicting patient prognosis than the RIFLE or AKIN criteria [290].

The diagnosis of acute kidney injury using serum creatinine levels in the RIFLE, AKIN, KDIGO, and ICA-AKI criteria
Various studies have validated the usefulness of the RIFLE, AKIN, and KDIGO criteria. But because these criteria were not developed for patients with cirrhosis, it is not clear whether these criteria can be directly applied to cirrhotic patients. Both the RIFLE and AKIN criteria include a decrease in urine volume in the definition of AKI. This could be a problem in the diagnosis of AKI in patients with cirrhosis because urine volume may decrease without a decrease in renal function, and the use of diuretics may affect the urine volume without changing the renal function in these patients [291]. Therefore, the International Club of Ascites (ICA) recently proposed the following definition of AKI: an increase in the sCr level of ≥0.3 mg/dL within 48 hours, or ≥50% from baseline within 7 days. Changes in urine volume are excluded from the definition [282]. In this definition, the baseline sCr level is defined as an sCr value obtained in the previous 3 months, when available. In patients without a prior sCr level, the sCr level on admission should be used as a baseline level [282]. In patients without a prior sCr level, a baseline level could be calculated by the reverse application of the Modification of Diet in Renal Disease (MDRD) formula (using an arbitrarily defined normal value for the glomerular filtration rate [GFR] of 75 mL/min) [289]. This is not recommended because sCr-based methods to estimate GFR (such as the MDRD formula) are not accurate in patients with cirrhosis [292]. Previous studies have shown that the efficacy of these methods for diagnosing AKI in cirrhosis patients is not precise [293]. In one study involving 373 patients admitted for liver cirrhosis and bacterial infection, AKI (as defined by the ICA criteria) was a significant predictor of 30-day mortality [294].
Hepatorenal syndrome: In advanced cirrhosis, aggravation of the effective hypovolemia by severe systemic and splanchnic vasodilatation leads to further potent activation of the renin-angiotensin and sympathetic nervous systems. Eventually, it causes a decrease in renal blood flow and potent renal vasoconstriction. In this condition, renal injury does not respond to the replacement of intravascular volume, and HRS develops. Although HRS in patients with cirrhosis is a reversible functional injury, structural injury in the glomerulus and/or renal tubule could be combined [295].
Similar to AKI, there have been several changes in the diagnostic criteria of HRS. In 1996, the ICA proposed diagnostic criteria for HRS [111], and these were updated in 2007 (Supplementary Table 1, 2) [263]. The main differences in the 2007 updated definition were as follows: 1) estimation of the creatinine clearance using 24-hour urine collection was excluded because of complexity and inaccuracy; 2) HRS can be diagnosed in patients with bacterial infection; 3) intravenous albumin infusion, rather than normal saline, should be used for volume replacement; and 4) minor criteria in the previous (1996) definition were excluded because of low sensitivity and specificity. A fixed sCr threshold of 2.5 mg/dL remained in the updated definition, which might delay the initiation of vasoconstrictors and albumin treatment and lead to a lower treatment response. A subgroup analysis that showed a lower treatment response rate in patients with a higher baseline sCr level supports this suggestion [296,297]. In 2015, the ICA proposed a new HRS definition, excluding a fixed sCr threshold (Table 10). Using these criteria, HRS is defined as AKI in patients with cirrhotic ascites that is not responsive to two consecutive days of diuretic withdrawal or plasma volume expansion with albumin (1 g/kg body weight) [282]. According to these criteria, vasoconstrictor and albumin treatment could be initiated at an earlier stage, before the sCr level reaches 2.5 mg/dL [282]. Because the overuse of albumin could lead to pulmonary edema, caution is needed [298].
The first guidelines for HRS were published in 1996. HRS was classified into two types according to the progression time. Type 1 HRS, rapidly progressive renal failure, was defined as a doubling of the sCr level (≥2.5 mg/dL) within 2 weeks. Type 2 HRS was defined as a moderate and slowly progressive renal failure (sCr level of 1.5-2.5 mg/dL), usually associated with refractory ascites [111]. In 2012, the ADQI group recommended that, in patients with type 2 HRS, those with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 (calculated using the MDRD-6 formula) for more than three months should be diagnosed with chronic kidney disease (CKD). The development of AKI in these patients should be classified as acute on CKD [299]. Based on these recommendation, renal dysfunction in cirrhotic patients would be classified as AKI, type 1 HRS, or type 2 HRS in patients without underlying renal disease, and AKI or type 1 HRS in patients with CKD or type 2 HRS [299]. However, an sCr-based estimation of renal function is not accurate in cirrhotic patients, and its accuracy is still unknown in the differentiation of CKD and type 2 HRS in patients with cirrhotic ascites. In addition, the clinical efficacy of the ADQI classification of renal dysfunction is not clear. Updated (2015) diagnostic criteria for HRS by the ICA clarify that patients with cirrhotic ascites and AKI can be diagnosed with HRS if they meet the criteria, without type classification [282].
In some cases of AKI, the differentiation of pre-renal azotemia, HRS, and acute tubular necrosis can be challenging. Several studies have suggested that biomarkers, such as neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), and liver-type fatty acid binding protein (L-FABP), could be helpful in the differentiation [300]. However, further data are required to confirm the usefulness of these biomarkers.
Prevention
A main strategy for preventing renal injury in cirrhotic patients is preventing a decrease in plasma volume or vasodilation. To prevent a decrease in plasma volume, doses of diuretics and non-absorbable disaccharides should be cautiously titrated. After LVP, intravenous albumin infusion is more effective than normal saline or dextran for the prevention of AKI [301]. In addition, avoidance of aminoglycosides or NSAIDs could be helpful for the prevention of acute tubular necrosis [302]. In patients with SBP, intravenous albumin infusion with antibiotics could prevent the development of HRS [106,228]. In patients with low ascitic protein (<1.5 g/dL), with renal dysfunction (sCr ≥1.2 mg/dL or BUN ≥25 mg/dL), or with serum Na <130 mEq/L, treatment with oral norfloxacin reduces the incidence of HRS and increases the three-month survival rate [247]. Pentoxifylline is more effective than corticosteroids for survival in patients with severe alcoholic hepatitis (Maddrey’s discriminant factor ≥32). A lower incidence of HRS in patients receiving pentoxifylline may point to a renal-protective effect [303]. However, in a previous large-scale, double-blinded, randomized controlled trial to compare the efficacy of prednisolone and pentoxifylline in patients with severe alcoholic hepatitis, treatment with pentoxifylline did not affect the incidence of mortality or AKI [304]. In some retrospective studies, rifaximin decreased the incidence of AKI and HRS in patients with cirrhotic ascites [249,305]. But in a double-blinded, randomized controlled trial to compare the efficacy of lactulose only and lactulose with rifaximin to treat hepatic encephalopathy, the combined treatment (lactulose with rifaximin) was more effective in improving hepatic encephalopathy and patient prognosis, although it did not affect the incidence of HRS [306].
[Recommendations]
1. In patients with liver cirrhosis, acute kidney injury is defined as an increase in serum creatinine of ≥0.3 mg/dL within 48 hours, or ≥1.5 times the baseline within 7 days (B1).
2. In patients with liver cirrhosis and ascites, hepatorenal syndrome is defined as no response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin (1 g/kg body weight), in absence of other potential causes of renal injury (B1).
3. In patients with spontaneous bacterial peritonitis, intravenous albumin infusion prevents hepatorenal syndrome development in those with high risk factor for hepatorenal syndrome (A1).
Treatment of acute kidney injury and hepatorenal syndrome in cirrhosis
General management
The therapeutic approach for AKI that accompanies cirrhosis depends on the cause of AKI, precipitating factors of renal damage, other organ dysfunction, and comorbid conditions. Management should be preceded by a process to verify these factors (Fig. 2). Functional impairment, which is not a renal parenchymal injury, can be reversed by elimination of the causative factors. It is necessary to first correct reversible triggering factors that may cause acute renal injury early in treatment. Proteinuria and hematuria must be identified to distinguish structural damage and the possibility of renal damage by nephrotoxic drugs or radiological contrast agents, which should be identified and discontinued. In addition, NSAIDs and vasodilators should be discontinued, and diuretics should be reduced or discontinued. In the first stage of AKI, if the plasma volume is inadequate, the patient should actively increase plasma volume by administering crystalloid fluid, albumin, and blood products. If a bacterial infection is suspected, antibiotics should be administered immediately. If sCr is recovered to within a 0.3 mg/dL increase in baseline in response to these primary treatments, it is necessary to check sCr for 2 to 4 days intervals during hospitalization, and at 2-4 week intervals after discharge for six months [262]. If the renal impairment is exacerbated to AKI stage 2 or 3 in spite of the initial treatment, or if the patient presents with AKI stage 2 or 3, diuretics should be discontinued. The patient should receive treatment for plasma volume expansion for 2 consecutive days together with 1 g/kg/day (up to 100 g/day) of albumin intravascular supply [263,307]. If there is no response to therapy and the patient complies with HRS, administration of a vasoconstrictor (such as terlipressin) with albumin should be considered [282]. A therapeutic approach based on these criteria is expected to help identify the type of AKI and to diagnose and treat the HRS slightly earlier, but it is still necessary to establish evidence through large-scale prospective clinical studies. The ICA has defined AKI progression, regression, and therapeutic response as no, partial, and complete response to therapy as follow (Table 11).
Pharmacological treatment
Albumin: Albumin accounts for 60% of normal plasma proteins. It has a negative charge, which exerts oncotic pressure that attracts sodium and water [308]. Therefore, administration of albumin may be a useful evaluation method for determining the relationship between AKI and insufficient plasma volume. In ICA, 1 g of albumin per kg of body weight (up to 100 g per day) is administered for 2 days, and it is used as an important index for judging the occurrence of HRS [282]. In addition, albumin has a free cysteine moiety (cys34) that gives it antioxidant and scavenging properties. It is capable of absorbing and removing proinflammatory cytokines, bacterial products, and radical oxygen species [309]. Administration of albumin improves renal blood flow in patients with AKI accompanied by acute decompensated aggravation of cirrhosis. This alleviates the secondary hyperactivity of the sympathetic nervous system [310]. A combination therapy of antibiotics and albumin for SBP is known to improve renal blood flow and the survival rate compared with antibiotic monotherapy [106,228,229]. In the case of cirrhotic patients with infectious diseases other than SBP, a combined treatment of antibiotics and albumin delayed the onset of AKI (compared with antibiotic monotherapy) in a randomized controlled trial of 193 patients [298]. However, there was no difference in the cumulative incidence of kidney injury or mortality rate, with a higher incidence of pulmonary edema (8.3%) among albumintreated group. Therefore, the albumin influsion should be cautiously used [298]. Although albumin alone cannot be improve HRS [311,312], albumin has anti-inflammatory and antioxidant effects that regulate immune responses and stabilize endothelial cells, helping the action of the vasoconstrictor [313].
Vasoconstrictors: Although vasoconstriction is central to the treatment of HRS, data on the efficacy of treating AKI with vasoconstrictors before the onset of HRS are still lacking [314].
Terlipressin
Terlipressin, a derivative of vasopressin, acts on V1 vasopressin receptors in the smooth muscle cells of the vascular wall to cause vasoconstriction. Terlipressin stabilizes the increased sympathetic nervous system and improves blood flow and perfusion to the kidney by moving the blood of the increased splanchnic circulation into other major organs through the constriction of the splanchnic vasculature in patients with liver cirrhosis.
In prospective randomized controlled trials, the combination of terlipressin and albumin showed improvement in renal function. Recovery of HRS was about 27-44%, showing a significant effect compared with albumin monotherapy [312,315,316]. However, in the above studies, the combination of terlipressin and albumin did not show an improvement in the survival rate compared with the control group. This was because many patients did not recover from HRS in the combination group. In the case of HRS associated with infection, sCr was reduced to less than 1.5 mg/dL in 12 of 18 patients who received the combination of terlipressin and albumin for 9 days. The improvement of infection and mean blood pressure were independent factors of renal function recovery, suggesting that elimination of systemic inflammation and circulatory disturbances are important factors in the recovery of renal function [297]. Although terlipressin is the best evidence-based treatment for HRS, it has not yet been approved for use in many countries, including the United States. Certain side effects of this drug (including abdominal pain and diarrhea) are reported to be as high as 30%. Although they are relatively rare, serious side effects (including ischemia of the extremities, hyponatremia, and arrhythmia like bradycardia) may occur [317]. There is a lack of evidence for the adequate dose and duration of therapy using terlipressin in the treatment of HRS. Generally, terlipressin is administered intravenously every 4-6 hours at 0.5-2.0 mg/dose, and increase up to 2 mg/dose every 4 hours if the sCr is not decreased by 25% after 3 days of drug administration. In patients with a therapeutic response, an increase in urinary output occurs within 12-24 hours of terlipressin administration, and an increase in the glomerular filtration rate appears slowly over several days. Maintenance therapy can be administered until the HRS is recovered, or up to 15 days. In a recent study comparing the administration of bolus doses and continuous infusion of terlipressin (to reduce ischemic side effects), the incidence of side effects was lower in continuous infusion group (62.1% and 35.3%, respectively), the total dose was also lower with continuous infusion group, while the response rate was similar (64.9% and 76.5%, respectively). Further study and consideration of drug administration methods are needed in the future [318]. Predicting the terlipressin therapy response is clinically important. An increase in mean blood pressure after treatment with terlipressin is a good prognostic factor. Severe hyperbilirubinemia (>10 mg/dL) and sCr >5 mg/dL at baseline are poor prognostic factors [296,316,319]. In a systematic review of randomized controlled trials including 739 patients, terlipressin was associated with a reduction in short-term mortality compared to placebo (OR, 0.65; 95% CI, 0.41-1.05) and compared to midodrine and octreotide (OR, 26.25; 95% CI, 3.07-224.21). After terlipressin treatment, 16% of patients (range, 5-20%) had recurrence of HRS after discontinuation of medication, and 8% (range, 4-22%) could not sustain the drug because of severe side effects [320].
Norepinephrine
Systemic vasoconstrictors like norepinephrine and midodrine increase mean arterial pressure and improve renal perfusion pressure. In a randomized comparison of norepinephrine and terlipressin in HRS patients, norepinephrine showed similar effects and side effects to terlipressin [321,322]. Combination therapy with norepinephrine and albumin was reported to be more effective than midodrine, octreotide, and albumin therapy [320,323]. Therefore, in countries where terlipressin is not approved for use (including those in North America), norepinephrine is recommended [148]. A recent systematic review of the literature suggests that norepinephrine has been effective in restoring HRS compared to placebo (OR, 4.17; 95% CI, 1.37-12.50), however, randomized controlled trials reporting norepinephrine effects are limited by small sized studies, and the evidence level to suggest norepinephrine use are weak. The use of this drug often requires monitoring of the heart in the intensive care unit. It is not preferred in regions where terlipressin is available, such as Korea. Norepinephrine is continuously infused at 0.5-3.0 mg per hour, and the dose is adjusted to increase the mean arterial pressure to 10 mmHg in the intensive care unit [324].
Midodrine and octreotide
Midodrine raises mean arterial pressure through the alpha-adrenergic effect. The combination of octreotide and albumin (a nonspecific vasoconstrictor of visceral blood vessels) significantly improves renal function in HRS [325-327], and some studies have shown significant survival improvement compared to non-treated groups [326,327]. In North America, this combination therapy is recommended for use in patients with HRS [148]. However, in a randomized controlled study, the improvement of renal function was lower than with terlipressin and albumin [328]. In a recent systematic review of the literature, terlipressin therapy was superior to midodrine and octreotide combination therapy (OR, 10.0; 95% CI, 1.5-50.0) [320]. Therefore, in countries where terlipressin is available (such as Korea), combination therapy with midodrine and octreotide is not considered as the primary treatment for HRS. Typically, a midodrine dose of 7.5-12.5 mg is given three times per day, with the goal of increasing mean arterial pressure by 15 mmHg. Octreotide is injected subcutaneously at a dose of 100-200 mg three times per day [324].
Non-pharmacological treatment:
Renal replacement therapy
If cirrhotic patients present with uremic symptoms, excessive fluid, refractory hyperkalemia, or metabolic acidosis despite medication, renal replacement therapy needs to be considered. These patients often have difficulty with hemodialysis due to hemodynamic instability, lack of effective plasma volume, and risk of bleeding. Continuous renal replacement therapy can be considered when conventional hemodialysis is not possible. In one study, a total of 102 patients with AKI awaiting liver transplantation were treated with renal replacement therapy, including continuous renal replacement therapy. Approximately 30% of patients receiving liver transplantation showed improvement, suggesting that renal replacement therapy in liver cirrhosis may be useful as a bridge treatment [329]. However, since renal replacement therapy itself does not lead to the recovery of HRS, renal replacement therapy without liver transplantation is not helful in improving the survival, and may result in prolonging a poor clinical outcome [299,330].
Transjugular intrahepatic portosystemic shunt
In year 1998, TIPS was performed in seven patients with type 1 HRS, reported that six of the seven patients had renal function recovery. However, the mean survival time was 4.7 months, and four mortalities among the seven patients were within 90 days [331]. Thus, safety and usefulness of TIPS is unclear. In early studies, TIPS was reported to reduce sCr and improve survival in patients with HRS [332,333]. Prior treatment with vasoconstrictors prior to the application of TIPS was also repored to be helpful in improving renal function [325]. TIPS was more effective in type 2 HRS than in type 1 HRS, as type 2 HRS shows relatively stable renal function [334]. However, there are no randomized controlled trials comparing TIPS with medical treatment including vasoconstrictors. TIPS may increase the risk of hepatic encephalopathy, reduce systemic blood pressure through systemic arterial relaxation, and possibly lower the renal perfusion pressure. Most studies reporting the usefulness of TIPS are based on selected patients with relatively stable liver function, so caution is needed in the interpretation and application of the results of TIPS studies in HRS [335,336].
Molecular adsorbent recirculating systems (MARS)
There are several kinds of extracorporeal therapies for liver disease, including molecular adsorbent recirculating systems (MARS), extracorporeal liver-assist devices, bio-artificial livers, bio-artificial liver support systems, and modular extracorporeal liver support systems. MARS is an albumin-assisted dialysis aid that helps maintain intestinal and systemic blood vessel relaxation by adsorbing and removing various cytokines and bacterial byproducts. MARS was designed to restore blood flow to the kidneys and improve renal function. In early studies, MARS caused a decrease in sCr levels [337,338]. However, decrease in sCr was not due to an improvement in renal function, but due to elimination of sCr by dialysis [339].
Liver transplantation: The only treatment that can improve the long-term survival rate in patients with HRS is liver transplantation. A reduction in renal function before transplantation may affect survival and increase complications following transplantation [265,340]. Recovery of renal function after liver transplantation is known to occur in about 50-75% of cases [341,342]. The duration of renal impairment before liver transplantation is an important factor in predicting renal function after transplantation. Renal replacement therapy more than 14 days prior to transplantation is associated with nonreversal of renal function, and increases risk by 6% per each day increase in renal replacement therapy [342]. This is likely related to structural changes of the kidney due to long-term ischemia. The liver and kidney dual transplantation is recommended when renal replacement therapy is given for more than 4 weeks before liver transplantation [299]. The prognosis of patients recovering from HRS after liver transplantation is good. The 6-month to 1-year survival rate is over 90% [342,343], and this is unrelated to the medication used for HRS before transplantation. If renal function is not restored after transplantation, the 1-year survival rate decreases to 60% [342]. Therefore, when a patient with HRS does not respond to medication, liver transplantation should be done as early as possible. There is no difference in the rate of renal function recovery between living-donor and cadaveric-donor liver transplantation [344,345].
[Recommendations]
1. In liver cirrhosis patients with acute kidney injury or hepatorenal syndrome, diuretics should be reduced or discontinued (A1).
2. In liver cirrhosis patients with acute kidney injury, restroring effective blood volume by albumin infusion is helpful in restoring renal function (A1).
3. The combination treatment of terlipressin and albumin is recommended for the improvement of renal function in hepatorenal syndrome (A1).
4. Where terlipressin is not available, the combination treatment of norepinephrine and albumin is recommended for the improvement of renal function in hepatorenal syndrome (A2).
5. The combination treatment of midodrine, octreotide, and albumin may also be considered in hepatorenal syndrome (B2).
6. The best treatment for hepatorenal syndrome is liver transplantation (A1).
OTHER COMPLICATIONS OF CIRRHOSIS
Hepatic hydrothorax
Hepatic hydrothorax (HH) is a complication of portal hypertension, characterized by a transudative pleural effusion in the absence of underlying cardiac or pulmonary disease. Its prevalence has been estimated to be 5-10% in cirrhosis patients [346].
Pathophysiology
The direct passage of fluid from the peritoneal to the pleural cavity through diaphragmatic defects is the accepted mechanism explaining most cases of HH [347]. Most diaphragmatic defects are <1 cm in size and are predominantly located on the right hemidiaphragm [348]. Malnutrition in cirrhosis seems to make thinning of the diaphragmatic muscle and formation of these defects. Additionary, negative intrathoracic pressure is thought to lead to the oneway directional flow of ascitic fluid from the abdominal cavity [349].
Clinical manifestation
Most patients have right-sided effusions, but a few patients present with left-sided or bilateral effusions. In one study, HH was right-sided in 70% of cases, left-sided in 18%, and bilateral in 12% [346]. When there is left-sided HH, possibility of tuberculosis, cancer, and pancreatic disease should be considered [350]. The pleural cavity is a restricted space, and smaller volumes of fluid (~500 mL) in the peritoneal space can make symptoms frequently [349]. Patients in whom pleural effusion is minimal may be asymptomatic, or they may have pulmonary symptoms of dyspnea, cough, chest discomfort, hypoxemia, or respiratory failure (usually associated with large pleural effusions).
Diagnosis
Chest radiography is used to diagnose the presence of pleural effusion, and thoracentesis is needed for the initial diagnosis of HH. Thoracentesis is performed to identify the cause of pleural effusion, to make sure the presence of infection, and to provide the relief of symptom. Thoracentesis can be performed without infusion of platelets or fresh frozen plasma [351]. Pleural fluid analysis should include protein, albumin, LDH, cell count, Gram stain, and culture examination. The nature of HH is transudate, and the diagnosis of uncomplicated HH is as follows: 1) a serum to pleural fluid albumin gradient (SPAG) > 1.1; 2) pleural fluid total protein < 2.5 g/dL, or pleural fluid/serum total protein ratio < 0.5; 3) pleural fluid/serum LDH ratio < 0.6; and 4) PMN < 250 cells/mm3 [352]. Spontaneous bacterial pleuritis (SBPL) is a infection of HH and requires prompt antibiotic therapy. When SBPL is suspected, diagnostic thoracentesis is essential. SBPL is diagnosed when PMN > 250 cells/mm3 with positive pleural fluid culture, or when PMN > 500 cells/mm3 with negative pleural fluid culture (without any evidence of pneumonia on chest X-ray) [353]. Symptoms of SBPL differ from fever and pleuritic chest pain to deteriorating encephalopathy or worsening of renal function. The microorganisms involved in SBPL are similar to those involved in SBP [354,355]. SBPL develops in 10–16% of patients with cirrhosis and HH, and is more common in patients with low total protein (<1.5 g/dL), low pleural fluid C3 complement levels, and more higher Child-Pugh score [354,355]. Over 50% of patients with ascites and SBPL do not develop concomitant SBP. In such patients who is suspected with infection but paracentesis is negative, thoracentesis is essential [353].
Treatment
The development of HH represents progression to decompensated cirrhosis and should warrant prompt consideration for liver transplantation [356]. Medical management of HH is similar to the management of ascites. Restriction of sodium intake with the administration of diuretics is effective in controlling HH, and therapeutic paracentesis is performed in cases of symptomatic dyspnea. Limiting pleural fluid removal to 1-2 L is recommended to decrease the risk of re-expansion pulmonary edema, although recent data suggest that larger volumes can be safely removed if no symptoms develop during the procedure, and end-expiratory pleural pressure remains below 20 cmH2O [357]. However, repeated thoracentesis is associated with an increased risk of infection, bleeding, and protein loss [351]. The standard of care for refractory HH is TIPS, with response rates of 70–80% [358-362]. However, severe liver dysfunction, poorly controlled hepatic encephalopathy, right-sided heart failure, pulmonary hypertension, and complete portal vein thrombosis are contraindications for TIPS in HH. Risk factors for increased mortality in patients receiving TIPS for HH include elevated baseline sCr, a MELD score > 15, and a poor response to TIPS [349].
Video-assisted thoracoscopy with pleurodesis is a potential treatment alternative for patients with refractory HH who are not eligible for TIPS, or who have failed to respond to TIPS [363,364]. However, video-assisted thoracoscopy appears to be inferior to TIPS and can have complications including fistula, empyema, and death. Therefore, it should only be considered in cases that are uncontrollable with medical treatment and TIPS [349]. Chest tube insertion should be cautious in HH and SBPL due to serious complications including empyema, hemothorax, pneumothorax, and HRS [365,366]. Since chest tube insertion is associated with high adverse events, and as most cases of SBPL respond to antibiotic therapy alone, a chest tube should not be placed in patients with SBPL unless they meet specific criteria (e.g., frank pus or pH < 7.2) [353,367]. Indwelling pleural catheter insertion can provide symptomatic relief until TIPS or transplantation, and can be performed. However, further studies are required to see the effectiveness of indwelling pleural catheters [367-370].
[Recommendations]
1. The development of hepatic hydrothorax should prompt consideration for liver transplantation. First-line therapy consists of dietary sodium restriction and diuretics (B1).
2. Transjugular intrahepatic portal-systemic shunt can be considered for refractory hepatic hydrothorax (B2).
3. Spontaneous bac terial pleuri t is is diagnosed when polymorphonuclear leukocyte > 250 cells/mm3 with positive pleural fluid culture, or when polymorphonuclear leukocyte > 500 cells/mm3 with negative pleural fluid culture without any evidence of pneumonia. Spontaneous bacterial pleuritis can be treated with appropriate antibiotics alone in most cases (B1).
Abdominal hernia in patients with cirrhotic ascites
Abdominal hernia (including umbilical, inguinal, and femoral hernia) is common in cirrhotic patients with ascites. Particularly, umbilical hernia is observed in up to 20% of these patients [371]. Abdominal hernia can be prevented by lowering intra-abdominal pressure with effective ascites control. For the prevention of umbilical hernia, an abdominal support belt may be helpful. Manual support is recommended to prevent abdominal hernia in situations of increased abdominal pressure, such as coughing or straining.
If large-volume of ascites is evacuated rapidly (e.g. due to LVPs, peritoneovenous shunt, or TIPS), the large intestine or omentum may be trapped in the hernia ring, and incarceration may occur. Trapped intestine can turn necrotic or perforate if manual reduction fails, and emergency surgery should be considered in this situation. Thus, patients with hernia should acknowledge the risk of developing incarceration.
Patients scheduled for liver transplantation may require hernia repair during or after transplantation. Patients who are not on a waiting list for liver transplantation require a careful decision for surgery. Strangulated hernia (which does not respond to manual reduction) needs emergency surgery, even in patients with decompensated liver function [372]. For patients with preserved liver function, surgical reduction can be considered (even for non-strangulated hernia) to improve quality of life [373,374].
If ascites exists before surgical repair, recurrence of hernia is very frequent (up to 73%), so ascites control is very important [375]. To control ascites before surgery, a multidisciplinary approach may be necessary, such as TIPS in addition to diuretic treatment [376]. To prevent the recurrence of hernia after surgery, all patients are advised to restrict their daily sodium intake to < 2 g (5 g of salt), and to minimize the use of sodium-rich intravenous fluid therapy.
[Recommendations]
1. Controlling ascites is important to reduce intra-abdominal pressure. This can prevent the occurrence of an abdominal hernia, or slow the worsening of a hernia (B1).
2. Strangulated hernia is an indication for emergent surgical repair (B1).
CONSIDERATIONS FOR DRUG USE IN CIRRHOTIC PATIENTS
The liver plays a central role in the absorption, distribution, and elimination kinetics of most drugs and many active or inactive drug metabolites. Impairment of liver function may have complex effects on drug clearance, biotransformation, and a drug’s pharmacokinetics. These changes can lead to alterations in various parameters affecting the efficacy or safety of drugs. Sometimes alterations increase levels of the bioavailable drug, causing normal drug doses to have toxic effects. Therefore, patients with hepatic dysfunction may be more sensitive to the effects, both desired and adverse, of several drugs. The main problem with drug use in patients with hepatic dysfunction is that physicians cannot define with precision the degree of impairment of liver function relevant to elimination of a particular drug in a given patient. Unfortunately, there is currently no single equivalent of the clearance creatinine test (as for renal disease) routinely available to clinicians to accurately determine what extent hepatic dysfunction will have on a drug’s pharmacokinetics. Moreover, there is no clear test to predict hepatic function with respect to the elimination capacity of specific drugs, and no general rules are available for modifying drug dosage in patients with hepatic dysfunction. It is important to have a solid understanding of changes to a drug’s pharmacokinetic properties, in combination with an assessment of a patient’s hepatic function in cirrhotic patients [377].
Effects of cirrhosis on drug metabolism
The liver plays a major role in drug metabolism. Metabolism is dependent on the metabolic capacity of the liver and hepatic blood flow. A number of significant pharmacokinetic changes are known to occur in cirrhotic patients. These often raise several concerns in using medications safely [378,379]. Hepatic clearance of drugs depends on the activity of drug-metabolizing enzymes, and clearance capacity is often reduced in cirrhotic patients. Cirrhosis may lead to the formation of portosystemic shunts, which are new blood vessels that divert blood from the abdominal viscera to the heart (bypassing the liver). As a result, a substantial fraction of the blood, which would normally reach the portal vein, flows through these shunts. This process decreases rates of drug metabolism. It also affects absorption, distribution, bioavailability, elimination, and cytochrome P450 metabolism due to hypoalbuminemia, portal hypertensive gastropathy, ascites, edema, and substantial renal blood flow reduction. In cirrhotic patients, the clinician should consider pharmacokinetic changes, the severity of liver disease, compromised metabolic pathways, and the administration route in choosing the type, dose, and administration interval of drugs. It is also important to consider changes in altered receptor sensitivity (i.e. pharmacodynamics, including tissue responsiveness to the pharmacological action). The pharmacodynamic responses to various drugs, and the frequency and pattern of adverse effects, are altered in cirrhotic patients. Clinically, the most important medications are sedatives (e.g. benzodiazepines), diuretics, and vasoconstrictors [378,380]. Cirrhotic patients usually have resistance or a diminished response to loop diuretics because of pharmacodynamic alterations. The natriuretic potency of furosemide is markedly reduced in decompensated patients with ascites [381].
Effects of transjugular intrahepatic portal-systemic shunt and portosystemic shunting on drug metabolism
TIPS and other surgical shunts (e.g. the Denver shunt) are performed to manage complications from portal hypertension. Patients who have undergone TIPS appear to develop changes in drug metabolism. For drugs with a high hepatic extraction, portosystemic shunting (both endogenous and iatrogenic) may reduce first-pass metabolism. This can increase oral bioavailability and decrease drug clearance in the liver [377,382]. Thus, if such drugs are administered orally to cirrhotic patients, the initial dose should be reduced according to the ratio of their hepatic extraction. Examples include beta-adrenergic blockers, calcium channel antagonists, cisapride and other prokinetic agents, antipsychotics, antianxiety and sedative agents, antiparkinson drugs, antidepressants, sumatriptan, certain statins (e.g. fluvastatin and lovastatin), and morphine. Cirrhotic patients with artificial portosystemic shunting are frequently found to have baseline QTc interval prolongation, likely reflecting an altered ventricular repolarization due to the portosystemic shunting of splanchnic-derived cardioactive substances into the systemic circulation [383]. Clinicians should avoid prescribing any medications known to prolong QTc in cirrhotic patients who have undergone TIPS (e.g., patients who are being prescribed a fluoroquinolone for SBP treatment or prophylaxis), as it can lead to potentially fatal ventricular arrhythmias [377].
Analgesics
In 2012, the prescription pattern of analgesics for cirrhotic patients registered with the Health Insurance Review Assessment Service was reported. Approximately 40.5% of 125,505 patients claimed reimbursement for at least one prescription for analgesics. This study showed that many cirrhotic patients are exposed to analgesics [384].
Acetaminophen
Acetaminophen is a widely used nonprescription analgesic and antipyretic medication for mild-to-moderate pain and fever. Although acetaminophen rarely induces hepatotoxicity by an idiosyncratic mechanism, it is an intrinsic hepatotoxin with a narrow safety margin [385]. This means there is little difference between the maximum daily dose and a potentially harmful dose. acetaminophen toxicity can result from either an acute overdose or from chronic overuse. The recommended dose of acetaminophen in adults is 650 to 1,000 mg every 4 to 6 hours, not to exceed 4,000 mg in a 24-hour period. Single doses of more than 150 mg/kg (or 7.5 g) in adults are considered potentially toxic, although the minimal dose associated with liver injury can range anywhere from 4 to 10 g [386]. The probability of an individual patient without pre-existing liver disease or concomitant alcohol consumption developing clinically important hepatotoxicity when acetaminophen dosing is limited to less than 4 g/day is exceedingly rare. The American Liver Foundation recommends patients not exceed 3 g of acetaminophen daily for any prolonged period of time, and suggests a maximum daily dose of 2–3 g for cirrhotic patients [382,387]. Generally, low-dose therapy is acceptable in most patients with chronic liver disease/cirrhosis. But chronic use should be avoided, and cirrhotic patients with ascites should be cautious of acetaminophen use.
Non-steroidal anti-inflammatory drugs
NSAIDs are associated with an increased risk of variceal/ulcer hemorrhage, impaired renal function, and the development of diuretic-resistant ascites in cirrhotic patients [388-390]. Thus, NSAIDs should generally be used with caution in cirrhotic patients. Another concern related to the use of NSAIDs in cirrhotic patients with ascites is that they diminish the natriuretic effects of diuretics, leading to impaired free water clearance and the worsening of ascites and edema. Use of NSAIDs should be considered when evaluating patients with apparent diuretic-resistant ascites. Most NSAIDs are highly protein-bound, usually to albumin, thereby increasing the free component of NSAID in the serum [391]. Some NSAIDs (e.g. diclofenac) have a significant hepatotoxic potential [377].
Selective COX-2 inhibitors are effective analgesics that are associated with a decreased incidence of gastrointestinal and renal toxicity. However, they have been associated with an increased incidence of adverse cardiovascular events. At present, available studies on the safety and efficacy of COX-2 selective inhibitors in cirrhotic patients are limited. One pilot study in humans included 28 patients with cirrhosis and ascites who were randomly assigned to receive celecoxib, naproxen, or placebo [390]. A significant reduction in the GFR, renal plasma flow, and urinary prostaglandin E2 excretion was observed in the group receiving naproxen but not celecoxib. Suppression of the diuretic and natriuretic response to furosemide was also observed in the group receiving naproxen but not celecoxib. Furthermore, naproxen, but not celecoxib, significantly inhibited platelet aggregation. The study evaluated only short-term treatment and involved only a small number of patients. Clearly, further studies are needed to address the use of COX-2 inhibitors in cirrhotic patients with ascites.
Cardiovascular drugs
NSBBs such as propranolol and nadolol have been shown to effectively reduce the risk of variceal bleeding and re-bleeding due to a reduction of portal pressure. This effect is mediated by several mechanisms acting on the hemodynamic alterations present in cirrhotic patients (e.g. a decrease in cardiac output via β1 receptors, and a splanchnic vasoconstriction through β2 receptors) [392]. NSBBs typically have a high rate of first-pass extraction by the liver, and lower bioavailability. In cirrhotic patients, impairment of hepatic blood flow can decrease the metabolism of high-extraction drugs, leading to significantly higher drug exposure. Therefore, careful dose monitoring is needed [377,393]. Recently, a growing body of evidence has shown that NSBBs can be harmful in end-stage liver cirrhosis. In 2010, a study by Sersté et al. demonstrated reduced survival in patients with refractory ascites who were treated with propranolol [120]. This study initiated debate among hepatologists on the appropriate use of NSBBs in patients with refractory ascites. Nearly half of the patients included in the study by Sersté et al. [119] received the high propranolol dose of 160 mg. In a consecutive cross-over study, propranolol treatment was found to be associated with increased risk for paracentesis-induced circulatory dysfunction in cirrhotic patients with refractory ascites. NSBBs can cause exacerbations in systemic hemodynamics due to a reduction of cardiac output and systemic hypotension, resulting in renal insufficiency [394]. But more recent studies investigating the effects of NSBB treatment in cirrhotic patients with ascites have reported contrary results [395,396]. According to the concept of risk-benefit stratification, careful monitoring of blood pressure and renal function should be performed to identify scenarios in which the NSBB dose should be reduced, or treatment discontinued, in patients with refractory ascites or SBP.
Generally, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (ARBs) appear to be relatively well tolerated in cirrhosis [397,398]. However, they should be avoided (even in low doses) in cirrhotic patients with ascites since they can induce arterial hypotension and renal failure [399,400].
Statins
Statins, which are lipid-lowering agents, undergo first-pass hepatic metabolism and are associated with elevations in liver enzymes. Given that cirrhotic patients are at risk of decreased hepatic clearance, there is concern that this patient population may be at higher risk for complications from statin therapy. However, emerging data from prospective studies suggest that statin therapy appears to be safe and effective in patients with chronic liver disease and compensated cirrhosis [401,402]. A large-scale population-based study and meta-analysis have demonstrated a beneficial effect of statins on the risk of hepatic decompensation and mortality in patients with compensated cirrhosis [403,404]. It is important that clinicians understand the potential benefits, side effects, and challenges of using statin therapy in patients with cirrhosis. This requires regular monitoring of liver function. Besides their lipid-lowering effects, statins also improve endothelial function by increasing the synthesis of nitric oxide, restoring the function of endothelial cells, and increasing the number of endothelial progenitor cells by decreasing the activation of inflammatory cells. In vitro and pre-clinical studies have also suggested a favorable impact of statins on hepatic inflammation, fibrosis, and cancer [405,406]. However, more clinical studies will be necessary to assess the benefits of statin use in cirrhosis, and to evaluate the best statin for different cirrhosis contexts (such as fibrosis or portal hypertension) [407]. The pharmacokinetics of statins in advanced cirrhotic patients with ascites have not been reported, and use in this setting has been discouraged [408].
Proton pump inhibitors
There has been growing concern about the possible overuse and long-term side effects of proton pump inhibitors (PPIs). Several studies have suggested that PPIs are associated with increased risk for SBP, Clostridium difficile infection, and other serious infections in cirrhotic patients [409-413]. PPI use alters the gut microbiota. For example, hypochlorhydria induced by PPI use may lead to small bowel bacterial overgrowth and bacterial translocation. This might subsequently be important in the development of minimal or overt hepatic encephalopathy [409,414]. From drug trials of satavaptan for ascites control, the confounder-adjusted HR of epatic encephalopathy for current PPI use versus non-use was 1.36 (95% CI, 1.01-1.84). The adjusted HR of SBP for current PPI use versus non-use was 1.72 (95% CI, 1.10-2.69). It is essential that clinicians are aware of the potential deleterious effects of long-term PPI use in cirrhotic patients, and that PPIs are used with caution [415].
[Recommendations]
1. In cirrhotic patients, especially those with ascites, the use of drugs may cause altered pharmacokinetics/pharmacodynamics, and changes in susceptibility to side effects. Therefore, clinical efficacy and safety of drugs should be assessed frequently (A1).
2. In cirrhotic patients, acetaminophen use should not exceed 2-3 g/day (A1).
3. In cirrhotic patients with ascites, non-steroidal anti-inflammatory drugs can exacerbate ascites, edema, and renal function. Therefore, the use of non-steroidal anti-inflammatory drugs requires attention (B1).
4. In cirrhotic patients with refractory ascites or spontaneous bacterial peritonitis, non-selective ß-blockers should be used with caution. Careful monitoring of blood pressure and renal function is necessary (B1).
5. In cirrhotic patients with ascites, angiotensin-converting enzyme inhibitors and angiotensin II antagonists can induce arterial hypotension and renal failure, so their use requires attention (B1).
6. In cirrhotic patients, proton pump inhibitors can increase the incidence of spontaneous bacterial peritonitis and hepatic encephalopathy. Careful attention should be given to their long-term use (B1).
Notes
Conflict of Interest
Potential conflicts of interest are as follows:
Yong-Han Paik: Consulted Bayer; received honoraria from BMS, Gilead, Bayer, MSD, and Chongkundang; received grants from Yu-han and Dong-A ST.
Yeon Seok Seo: Received honoraria from Gilead, Dong-A ST, Il-dong, Hanmi, MSD, and Yuhan.
Moon Young Kim: Received grants from Alfa Wassermann, Samjin, and Yuhan; received honoraria from BMS, Gilead, and Dong-A ST; consulted Gilead.
Jun Yong Park: Received honoraria from BMS, Gilead, Yuhan, Dong-A ST, Hanmi, Celtrion, and CJ; received grants from Hanmi, Abbvie, Gilead, and Norvatis.
Ki Tae Suk: Nothing to disclose.
Do Seon Song: Received honoraria from BMS and Celtrion; consulted Samil.
Dong Hyun Sinn: Received honoraria from Gilead, Yuhan, Dong-A ST, and Celtrion; received a grant from Dong-A ST; consulted Abbvie and Bayer.
Jeong-Hoon Lee: Nothing to disclose.
Soung Won Jeong: Received honoraria from BMS and Hanmi; received grants from BMS, Samil, and Hanmi.
Young Kul Jung: Received honoraria from BMS, Dong-A ST, and Daewoog; received grants from Ferring, Sillajen, Dong-A ST, Dae-woong, Chongkundang, Ildong, and BMS.
SUPPLEMENTAL MATERIAL
Supplementary materials are available at Clinical and Molecular Hepatology website (http://www.e-cmh.org)
Diagnostic criteria for HRS of the International Club of Ascites in 1996 [111]
Diagnostic criteria for HRS of the International Club of Ascites in 2007 [263]
Abbreviations
ADA
adenosine deaminase
ADQI
Acute Dialysis Quality Initiative
AFB
acid-fast bacilli
ALT
alanine aminotransferase
AKI
acute kidney injury
AKIN
Acute Kidney Injury Network
BCAA
branched-chain amino acid
CART
cell-free and concentrated ascites reinfusion therapy
CEA
carcinoembryonic antigen
CKD
chronic kidney disease
CI
confidence interval
eGFR
estimated glomerular filtration rate
ESBL
extended-spectrum beta-lactamase
GFR
glomerular filtration rate
HCC
hepatocellular carcinoma
HH
hepatic hydrothorax
HR
hazard ratio
HRS
hepatorenal syndrome
ICA
International Club of Ascites
KASL
The Korean Association for the Study of the Liver
KDIGO
Kidney Disease Improving Global Outcomes
LDH
lactate dehydrogenase
LVP
large-volume paracentesis
MARS
molecular adsorbent recirculating system
MDRD
Modification of Diet in Renal Disease
MELD
Model for End-Stage Liver Disease
NSAIDs
non-steroidal anti-inflammatory drugs
NSBBs
Non-selective beta-blockers
OR
odds ratio
PMN
polymorphonuclear leukocyte
PPIs
proton pump inhibitors
RBCs
red blood cells
SAAG
serum-ascites albumin gradient
SBP
spontaneous bacterial peritonitis
SBPL
spontaneous bacterial pleuritis
sCr
serum creatinine
SIADH
syndrome of inappropriate antidiuretic hormone secretion
SPAG
serum to pleural fluid albumin gradient
TIPS
transjugular intrahepatic portal-systemic shunt