Hepatitis C virus (HCV) causes various liver diseases including acute hepatitis, chronic hepatitis, health-holders, liver cirrhosis and hepatocellular carcinoma.1 Acute hepatitis C progresses to chronic hepatitis from 75 to 85% and cirrhosis after 20-25 years later. In patients with HCV-positive cirrhosis, the annual incidence of hepatocellular carcinoma has been reported as 5% approximately.1
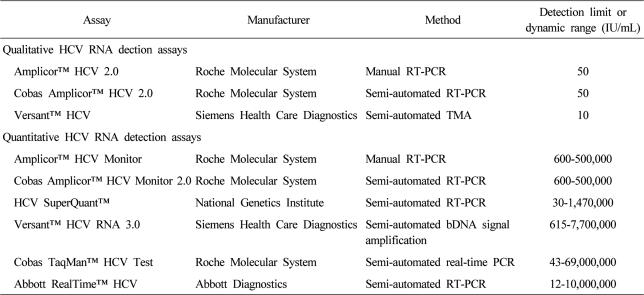
The current standard antiviral treatment with peginterferon and ribavirin combination therapy is effective in about 50% of patients with chronic hepatitis C. It is assumed that achievement of sustained virological response (SVR) after the combination therapy, confirmed by means of commercial HCV RNA detection assays is regarded as a cure for chronic hepatitis C (). It is associated with histological improvement, reduced the risk of hepatocellular carcinoma and liver related mortality, and improved quality of life.
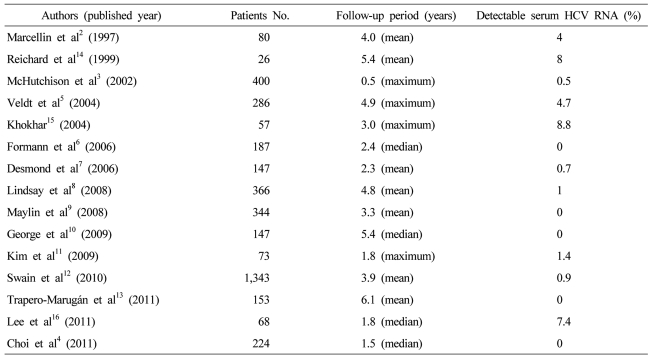
For the respect of the durability of SVR, several studies have been reported. Marcellin et al2 reported that an SVR after antiviral therapy was associated with long-term biochemical and virological responses as well as histologic improvement. Eighty-patients who had SVR after interferon-alpha monotherapy, mean follow-up of 4 years showed that 96% had undetectable serum HCV RNA. In a large-scale clinical trials, 7 (2%) among 400 sustained virological responder had detectable hepatic HCV RNA during follow-up; 5 have been followed and 2 (0.5%) were reappearance of serum HCV RNA at 12 months after therapy.3 Many other reports including the study of Choi et al4 confirmed that late relapse is rare after achievement of an SVR with completion of interferon without or with ribavirin therapy ().5-13 On the other hand, earlier reports showed higher rate of late relapse.14-16 These discrepancies may be resulted from use of HCV polymerase chain reaction (PCR) assays with varying degrees of sensitivity and a range of patient populations. As a result, the true durability of the response remains unclear, and the optimal follow-up for patients who have achieved an SVR is unknown.
Even then achievement of an SVR, there are many evidences that HCV can persist and replicate in peripheral blood mononuclear cells, hepatic tissue and other organs including kidney, heart, pancreas, intestine, adrenal gland, lymph node and gallbladder in spite of still undetectable serum HCV RNA.3,17-21 It is so called "occult HCV", which can be reservoir of reappearance HCV RNA in serum3,21 and thus play a role in the persistence and reactivation of infection. Highly sensitive reverse transcription and nested PCR assays detected residual HCV RNA in case of occult HCV. However, these assays are not currently standard diagnostic procedures and whether these findings actually represent a replication-competent virus or have any clinical significance is not clear. The immunologic mechanism of HCV recurrence after SVR is also not well investigated. Quiroga et at22 reported HCV-specific cellular immune responses are stronger in occult HCV infection than in chronic hepatitis C and it would be the cause of the rare incidence of late relapse.
For the factors affecting the durability of SVR, no distinct pattern of baseline characteristics could be identified. Several studies with small number of patients reported the transfusion, injection drug use or dose reduction as a risk factor. However, it was unknown whether these cases reflect relapse or re-infection because of the paucity of paired samples.
Although there is reappearance of serum HCV RNA in approximate 1% of patients with an SVR, individuals who have achieved an SVR should have a single additional follow-up HCV RNA measurement performed to ensure that the negative HCV RNA result at 6 months posttreatment was not a false negative possibly due to faulty sample collection. If the second posttreatment HCV RNA measurement is negative, the SVR appears to be durable and a reliable sign of cure after antiviral therapy in chronic hepatitis C. However, it is recommended to follow up serum HCV RNA for a long time after an SVR for those individuals who show intermittent viral breakthrough during treatment or who may be considered at risk for reinfection or late relapse.
References
1. Williams R. Global challenges in liver disease. Hepatology 2006;44:521–526.
16941687.
2. Marcellin P, Boyer N, Gervais A, Martinot M, Pouteau M, Castelnau C, et al. Long-term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon-alpha therapy. Ann Intern Med 1997;127:875–881.
9382365.
3. McHutchison JG, Poynard T, Esteban-Mur R, Davis GL, Goodman ZD, Harvey J, et al. Hepatic HCV RNA before and after treatment with interferon alone or combined with ribavirin. Hepatology 2002;35:688–693.
11870385.
4. Choi SB, Lee YJ, Lee JI, Song YJ, Choi BJ, Kim JH, et al. Durability of a sustained virological response in chronic hepatitis C patients treated with pegylated interferon alfa and ribavirin. Korean J Hepatol 2011;17:183–188.
22102384.
5. Veldt BJ, Saracco G, Boyer N, Cammà C, Bellobuono A, Hopf U, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut 2004;53:1504–1508.
15361504.
6. Formann E, Steindl-Munda P, Hofer H, Jessner W, Bergholz U, Gurguta C, et al. Long-term follow-up of chronic hepatitis C patients with sustained virological response to various forms of interferon-based anti-viral therapy. Aliment Pharmacol Ther 2006;23:507–511.
16441471.
7. Desmond CP, Roberts SK, Dudley F, Mitchell J, Day C, Nguyen S, et al. Sustained virological response rates and durability of the response to interferon-based therapies in hepatitis C patients treated in the clinical setting. J Viral Hepat 2006;13:311–315.
16637861.
8. Lindsay K, Manns MP, Gordon SC, Pockros P, Haussinger D, Hadziyannis SJ, et al. Clearance of HCV at 5 year follow-up for peginterferon alfa-2b ± ribavirin is predicted by sustained virologic response at 24 weeks post treatment. [Abstract]. Gastroenterology 2008;134:A772.
9. Maylin S, Martinot-Peignoux M, Moucari R, Boyer N, Ripault MP, Cazals-Hatem D, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology 2008;135:821–829.
18593587.
10. George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology 2009;49:729–738.
19072828.
11. Kim CH, Park BD, Lee JW, Kim YS, Jeong S, Lee DH, et al. Durability of a sustained virologic response in combination therapy with interferon/peginterferon and ribavirin for chronic hepatitis C. Korean J Hepatol 2009;15:70–79.
19346787.
12. Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology 2010;139:1593–1601.
20637202.
13. Trapero-Marugán M, Mendoza J, Chaparro M, González-Moreno L, Moreno-Monteagudo JA, Borque MJ, et al. Long-term outcome of chronic hepatitis C patients with sustained virological response to peginterferon plus ribavirin. World J Gastroenterol 2011;17:493–498.
21274379.
14. Reichard O, Glaumann H, Frydén A, Norkrans G, Wejstål R, Weiland O. Long-term follow-up of chronic hepatitis C patients with sustained virological response to alpha-interferon. J Hepatol 1999;30:783–787.
10365802.
15. Khokhar N. Late relapse in chronic hepatitis C after sustained viral response to interferon and ribavirin. J Gastroenterol Hepatol 2004;19:471–472.
15012792.
16. Lee JE, Yoon NR, Kim JD, Song MJ, Kwon JH, Bae SH, et al. Durability of sustained virologic response in chronic hepatitis C: analysis of factors related to relapse after sustained virologic response with peginterferon plus ribavirin combination therapy. Korean J Gastroenterol 2011;57:173–179.
21519165.
17. Pham TN, MacParland SA, Mulrooney PM, Cooksley H, Naoumov NV, Michalak TI. Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J Virol 2004;78:5867–5874.
15140984.
18. Castillo I, Rodríguez-Iñigo E, Bartolomé J, de Lucas S, Ortíz-Movilla N, López-Alcorocho JM, et al. Hepatitis C virus replicates in peripheral blood mononuclear cells of patients with occult hepatitis C virus infection. Gut 2005;54:682–685.
15831916.
19. Pham TN, Macparland SA, Coffin CS, Lee SS, Bursey FR, Michalak TI. Mitogen-induced upregulation of hepatitis C virus expression in human lymphoid cells. J Gen Virol 2005;86:657–666.
15722526.
20. Radkowski M, Gallegos-Orozco JF, Jablonska J, Colby TV, Walewska-Zielecka B, Kubicka J, et al. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology 2005;41:106–114.
15619235.
21. Lee WM, Polson JE, Carney DS, Sahin B, Gale M Jr. Reemergence of hepatitis C virus after 8.5 years in a patient with hypogammaglobulinemia: evidence for an occult viral reservoir. J Infect Dis 2005;192:1088–1092.
16107964.
22. Quiroga JA, Llorente S, Castillo I, Rodríguez-Iñigo E, López-Alcorocho JM, Pardo M, et al. Virus-specific T-cell responses associated with hepatitis C virus (HCV) persistence in the liver after apparent recovery from HCV infection. J Med Virol 2006;78:1190–1197.
16847959.