| Korean J Hepatol > Volume 17(3); 2011 > Article |
See the commentary-article "Durability of antiviral therapy for chronic hepatitis C after achieving sustained virological response" on page 180.
ABSTRACT
Background/Aims
The reappearance rates of hepatitis C virus (HCV) RNA after a sustained virological response (SVR) have been reported to be 1-2%. We investigated the reappearance rate of HCV RNA after SVR in chronic hepatitis C (CHC) patients treated with pegylated interferon (PEG-IFN) and ribavirin.
Methods
In total, 292 CHC patients who achieved an SVR after PEG-IFN and ribavirin treatment were included. They were treated with subcutaneous injections of either PEG-IFN-α 2a or 2b plus ribavirin orally. Liver function tests and qualitative HCV RNA assays were performed every 6 months during the follow-up period after an SVR.
Results
Among the 292 patients, 224 (genotype 1, 92; genotype non-1, 132) were followed up for more than 6 months after SVR. These 224 patients were aged 48.1±11.5 years (mean±SD), and 129 of them were male. The median follow-up duration was 18 months (range 6-60 months). The reappearance rate of HCV RNA during follow-up was 0%. Two patients who achieved an SVR developed hepatocellular carcinoma during the follow-up period.
Globally, it has been estimated that 170 million people are chronically infected with the hepatitis C virus (HCV), and 3 to 4 million are infected each year.1,2 The HCV is a major public health problem and a leading cause of chronic liver disease.3 Natural history studies indicate that 55% to 85% of individuals who develop acute hepatitis C will remain HCV-infected.4-6 The risk of developing cirrhosis ranges from 5% to 25% over periods of 25 to 30 years.7,8
The currently recommended therapy for chronic HCV infection is the combination of pegylated interferon (PEG-IFN) and ribavirin.9 The sustained virological response (SVR) rates in patients treated with PEG-IFN and ribavirin are 50% in HCV genotype 1 and 80-90% in HCV genotype 2 or 3.9-12 The achievement of the SVR in patients with chronic hepatitis C (CHC) has been associated with improvements in liver histology as well as a reduced risk of hepatocellular carcinoma (HCC) and liver-related mortality.13-15
Previous studies reported that the SVR after PEG-IFN and ribavirin combination therapy was maintained up to 99-100% during the long-term follow-up.16-20 However, a Korean study recently reported that the reappearance rate of HCV RNA after SVR was as high as 11%.21 Therefore, we investigated the reappearance rate of HCV RNA after SVR in CHC patients treated with PEG-IFN and ribavirin.
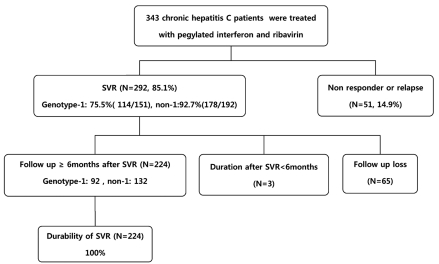
Three hundred forty three consecutive patients with CHC were treated with PEG-IFN and ribavirin at Paik Hospital, Busan, Korea, between April 2004 and December 2008. Among them, 292 patients (85.1%) with SVR were included in this study.
They were treated with subcutaneous injections of either PEG-IFN-α 2a (Pegasys®; F. Hoffmann-La Roche, Ltd., Basel, Switzerland), at a dose of 180 µg/week or PEG-IFN-α 2b (Peg-Intron®; Schering Plough Corp., Kenilworth, NJ), at a dose of 1.5 µg/kg/week and ribavirin orally. The ribavirin dose was determined according to the HCV genotype and the patients' body weight, as follows: dose of 1,000 mg/day (for patients weighing ≤75 kg) or 1,200 mg/day (for patients weighing >75 kg) in genotype 1 and 800-1,000 mg/day in genotype non-1. The standard treatment duration was 48 weeks in infections with the HVC genotype 1 and 24 weeks in those with the genotype non-1.
For the quantitative HCV-RNA assay, before February 2009, we used the Cobas Amplicor HCV Monitor Version 2.0 (Roche diagnostic, IN, USA), with the lower limit of detection of 600 IU/mL. From February 2009, we used the Cobas Ampliprep/Cobas TaqMan system (Roche Molecular Systems, Pleasanton, CA), with the lower limit of detection of 15 IU/mL. The HCV RNA was measured at baseline (i.e. before treatment) and during the treatment, at weeks 4 and 12. To assess the efficacy of the treatment, the qualitative HCV RNA assay (Cobas Amplicor HCV Test Version 2.0, Roche diagnostic, IN, USA, lower limit of detection, 50 IU/mL) was performed at the end of treatment and 24 weeks after completing the therapy. HCV genotyping was performed in all patients before treatment initiation, using the INNO-LiPA HCV II kit (Bayer Diagnostics, Emeryville, CA). The dose of PEG-IFN was reduced to 75% of the initial dose if the neutrophil count decreased under 750/mm3 or the platelet count were under 50,000/mm3, the dose was reduced to 50% if there was no improvement of cytopenia, and the treatment with PEG-IFN was discontinued if the neutrophil count further decreased under 500/mm3 or the platelet count decreased under 30,000/mm3. Ribavirin was reduced stepwise from the initial dose to 600 mg if the hemoglobin decreased under 10 g/dL, and was discontinued if the hemoglobin further decreased under 8 g/dL. When medication-related adverse effects occurred, such as flu-like symptoms, depression or insomnia, non steroidal anti-inflammatory drugs, anti-depressant and hypnotics were administered to improve the patients' symptoms. During the follow-up period after SVR, qualitative HCV RNA assays and liver function tests were performed every 6 months. We called the patients lost to follow-up within the past one year and asked for checking their liver function and HCV RNA test.
Rapid virological response was defined as HCV RNA negative at treatment week 4 by a sensitive PCR based quantitative assay. Early virological response was defined as qualitative HCV RNA negative or a reduction from baseline HCV RNA level of 2 log10 IU/mL at week 12. End of treatment response and SVR were defined, respectively, as a negative qualitative HCV RNA level at the end of treatment and after 24 weeks of untreated follow-up.
Relapse was defined as reversion to HCV RNA positive status in patients who had an undetectable HCV RNA level at the end of treatment, and reappearance was defined as HCV RNA positive after SVR.
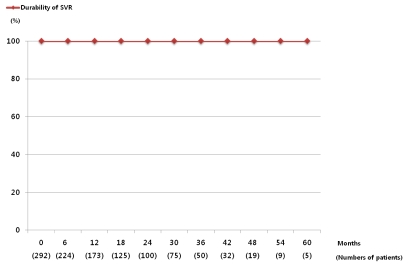
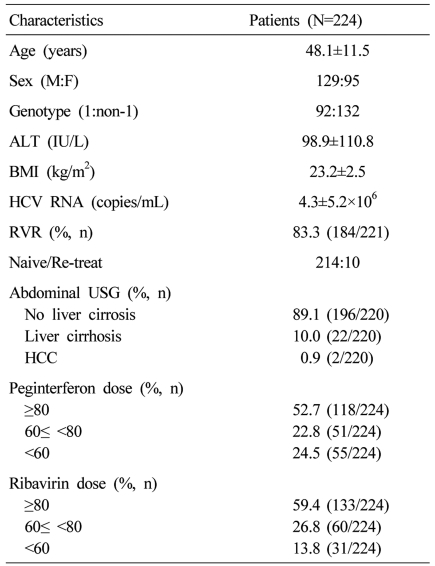
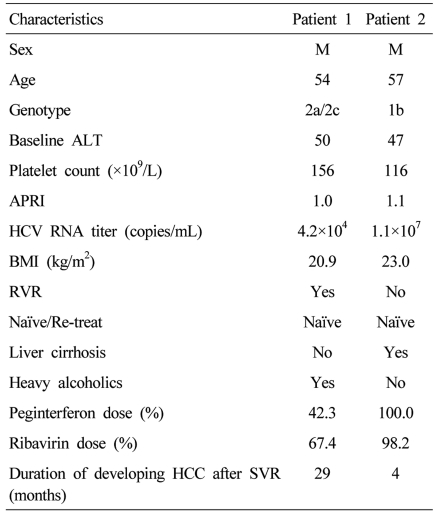
Among the 343 patients treated with PEG-IFN and ribavirin, the numbers of genotype 1 and non-1 patients were 151 and 192, respectively. Three hundred thirty eight patients (98.5%) achieved the end of treatment response and 292 patients (85.1%) achieved the SVR. The SVR was 75.5% (114/151) in genotype 1 and 92.7% (178/192) in genotype non-1. The numbers of patients who were followed-up with HCV RNA assays and liver function tests for more than 6 months after SVR were 224. Sixty five patients were lost to follow-up. The remaining three patients were less than 6 months of follow-up period, so they didn't perform HCV RNA assays. These 224 patients were actually followed-up for a median period of 18 months (range 6-60 months). The baseline characteristics and treatment doses of the patients were shown in Table 1. During the follow-up period, ALT increased in 149 (66.5%) and was maintained within normal limits in 75 (33.5%). Pre-treatment abdominal ultrasonography was performed in 220 patients, 22/220 (10%) had liver cirrhosis and 196/220 (89.1%) did not. Two patients already had HCC. SVR was maintained in all patients (n=224, 100%) during the follow-up period (Fig. 1). If SVR was achieved, there was no relationship between reappearance and the dose of either PEG-IFN or ribavirin. There were two patients who achieved the SVR but developed HCC during the follow-up period. Among them, one had liver cirrhosis before the treatment, the other patients with HCC was a 54-year-old heavy alcoholic. He did not have liver cirrhosis radiologically, but his aspartate aminotransferase-to-platelet ratio index (APRI) was 1.0 (Table 2).
Figure 2 shows the numbers of patients who were followed up after SVR and the durability. SVR was maintained in all patients during the follow-up period, therefore there was no control group who had a reappearance.
The standard therapy for HCV infection is the combination of PEG-IFN and ribavirin. According to previous studies, SVR was maintained up to 99-100%, and the achievement of the SVR was considered as cure.9,16-20
However, according to a recent Korean study, 73 out of 156 patients treated successfully with interferon/peginterferon and ribavirin achieved the SVR. During the follow-up period of those 73 patients with SVR, the HCV RNA reappeared in 8 patients (11%). In this Korean study, patients were enrolled from January 2002 to October 2008, and the serum HCV RNA was measured quantitatively by real time polymerase chain reaction (RT-PCR) (ABI prism system, Applied Biosystems, USA).21 However, the RT-PCR assay using the ABI prism system was used only in the past 5 years, hence in patients who were treated earlier, the HCV may be quantitatively measured by the HCV RNA assay with lower sensitivity and higher limit of detection than RT-PCR assay using ABI prism system. Therefore, some patients who had small quantities of HCV RNA, which could be detected with the current sensitive HCV RNA assay, may be assessed that they achieved the SVR. Then, if the HCV RNA assay with higher sensitivity was used during the follow-up period, the HCV RNA could probably be detected in serum. This may be the cause for the higher reappearance rate detected in that study, compared to previous studies. Among the 8 patients who had reappearance of HCV in that study, only one patient had persistent viremia, 5 patients had transiently positive HCV RNA, and 2 patients were followed-up.21 It is possible that the transiently positive HCV RNA result may be false positive. Therefore, the reappearance rate can be considered as 1.4% (1/73). In a recent study, transient low levels of HCV RNA at single time points were detected in some serum samples during the follow-up, but they were all undetectable on retests.19 Therefore, the authors reported that these transient positive tests probably represented false positive results.20 In our study, we also experienced one transient postive HCV RNA result which was undetectable on retest. It is not known whether patients who become HCV RNA detectable during follow-up experienced reinfection or reappearance. Another Korean study recently reported that the reappearance rate after SVR was 7.4% (5/68). This higher reappearance rate also may be due to using HCV RNA assay with lower sensitivity in early treatment period.22
In our study, among the 292 patients who achieved the SVR in response to PEG-IFN and ribavirin combination therapy, 224 were followed up for a median period of 18 months (range 6-60 months). SVR was maintained in all patients (n=224, 100%), including patients who were treated with decreased doses of PEG-IFN or ribavirin, therefore the durability of SVR was as high as in previous studies. When patients were treated with decreased doses of PEG-IFN or ribavirin, they had lower SVR.23 After achievement of the SVR, however, we found no relationship between the treatment dose and reappearance. This suggests that, after achievement of the SVR, intensive follow-up is not needed in patients treated with lower doses of PEG-IFN or ribavirin than in patients who received sufficient doses.
In patients with CHC, achievement of the SVR has been associated with improvements in liver histology, as well as reduced risk of HCC and liver-related mortality. Similarly, in patients with HCV-related cirrhosis, achievement of the SVR after IFN therapy was associated with reduction of liver-related mortality lowering both the risk of complications and HCC development.13-15,24-26 However, in patients with advanced fibrosis or cirrhosis, achievement of the SVR cannot prevent completely the risk of HCC.23,27,28
In our study, there were two patients who achieved the SVR but developed HCC during the follow-up period. Among them, one had liver cirrhosis before the treatment, so liver may undergo irreversible changes, leading to an elevated risk for carcinogenesis even when the HCV RNA has been disappeared in serum. The other patient was a alcoholic and showed APRI 1.0 that might suggest hepatic fibrosis. Thus, significant fibrosis and persistent liver damage due to alcohol were thought to be related with development of HCC, even when the HCV RNA was undetectable. Therefore, screening of HCC, with abdominal ultrasound and alfa-fetoprotein, are needed in patients with chronic hepatitis C with advanced fibrosis, as well as liver cirrhosis, even if they achieved the SVR. Future studies should identify the degree of liver fibrosis susceptible to increase the risk for development of HCC.
To summarize, previous studies reported that SVR after PEG-IFN and ribavirin combination therapy was maintained up to 99-100% during the long-term follow-up.16-20 Our study showed that SVR was durable in all the CHC patients for a median follow-up of 18 months. However, screening test for HCC should be needed in the patients with SVR, particularly advanced fibrosis or cirrhosis.
REFERENCES
1. World Health Organization. Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat 1999;6:35-47. 10847128.


2. Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010;51:1176-1184. 20187106.


4. Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis 2005;9:383-398. vi. 16023972.


5. Strader DB, Seeff LB. The natural history of chronic hepatitis C infection. Eur J Gastroenterol Hepatol 1996;8:324-328. 8781898.


6. Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology 2002;36(5 Suppl 1):S1-S2. 12407571.


7. Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002;36(5 Suppl 1):S35-S46. 12407575.


8. Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 2000;132:296-305. 10681285.


9. Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335-1374. 19330875.


10. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958-965. 11583749.


11. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-982. 12324553.


12. Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140:346-355. 14996676.


13. Berenguer J, Alvarez-Pellicer J, Martín PM, López-Aldeguer J, Von-Wichmann MA, Quereda C, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology 2009;50:407-413. 19575364.


14. George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology 2009;49:729-738. 19072828.



15. Hung CH, Lee CM, Lu SN, Wang JH, Hu TH, Tung HD, et al. Long-term effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. J Viral Hepat 2006;13:409-414. 16842444.


16. Desmond CP, Roberts SK, Dudley F, Mitchell J, Day C, Nguyen S, et al. Sustained virological response rates and durability of the response to interferon-based therapies in hepatitis C patients treated in the clinical setting. J Viral Hepat 2006;13:311-315. 16637861.


17. Formann E, Steindl-Munda P, Hofer H, Jessner W, Bergholz U, Gurguta C, et al. Long-term follow-up of chronic hepatitis C patients with sustained virological response to various forms of interferon-based anti-viral therapy. Aliment Pharmacol Ther 2006;23:507-511. 16441471.


18. Chavalitdhamrong D, Tanwandee T. Long-term outcomes of chronic hepatitis C patients with sustained virological response at 6 months after the end of treatment. World J Gastroenterol 2006;12:5532-5535. 17006994.



19. Giannini EG, Basso M, Savarino V, Picciotto A. Sustained virological response to pegylated interferon and ribavirin is maintained during long-term follow-up of chronic hepatitis C patients. Aliment Pharmacol Ther 2010;31:502-508. 19925499.


20. Swain MG, Lai MY, Shiffman ML, Cooksley WG, Zeuzem S, Dieterich DT, et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology 2010;139:1593-1601. 20637202.


21. Kim CH, Park BD, Lee JW, Kim YS, Jeong S, Lee DH, et al. Durability of a sustained virologic response in combination therapy with interferon/peginterferon and ribavirin for chronic hepatitis C. Korean J Hepatol 2009;15:70-79. 19346787.


22. Lee JE, Yoon NR, Kim JD, Song MJ, Kwon JH, Bae SH, et al. Durability of sustained virologic response in chronic hepatitis C: analysis of factors related to relapse after sustained virologic response with peginterferon plus ribavirin combination therapy. Korean J Gastroenterol 2011;57:173-179. 21519165.


23. McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 2002;123:1061-1069. 12360468.


24. Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology 2007;45:579-587. 17326216.


25. Cammà C, Giunta M, Andreone P, Craxì A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: an evidence-based approach. J Hepatol 2001;34:593-602. 11394661.


26. Cardoso AC, Moucari R, Figueiredo-Mendes C, Ripault MP, Giuily N, Castelnau C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol 2010;52:652-657. 20346533.


Figure 2
Durability of the SVR in the CHC patients treated with PEG-IFN plus ribavirin. The numbers in parentheses represent the numbers of patients who were followed up, which was 224 for a median duration of 18 months (range 6-60 months). The SVR was maintained in 100% of the patients during the follow-up.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




