The clinical features of drug-induced liver injury observed through liver biopsy: focus on relevancy to autoimmune hepatitis
Article information
Abstract
Background/Aims
Accurate diagnosis of drug-induced liver injury (DILI) is difficult without considering the possibility of underlying diseases, especially autoimmune hepatitis (AIH). We investigated the clinical patterns in patients with a history of medication, liver-function abnormalities, and in whom liver biopsy was conducted, focusing on accompaniment by AIH.
Methods
The clinical, serologic, and histologic findings of 29 patients were compared and analyzed. The patients were aged 46.2±12.8 years (mean±SD), and 72.4% of patient were female. The most common symptom and causal drug were jaundice (58.6%) and herbal medications (55.2%), respectively.
Results
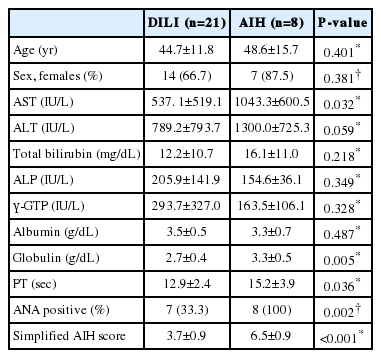
Aspartate aminotransferase (AST), alanine aminotransferase, total bilirubin, alkaline phosphatase, and γ-glutamyl transpeptidase levels were 662.2±574.8 U/L, 905.4±794.9 U/L, 12.9±10.8 mg/dL, 195.8±123.3 U/L, and 255.3±280.8 U/L, respectively. According to serologic and histologic findings, 21 cases were diagnosed with DILI and 8 with AIH. The AIH group exhibited significantly higher AST levels (537.1±519.1 vs. 1043.3±600.5 U/L), globulin levels (2.7±0.4 vs. 3.3±0.5 g/dL), and prothrombin time (12.9±2.4 vs. 15.2±3.9 s; P<0.05). Antinuclear antibody was positive in 7 of 21 cases of DILI and all 8 cases of AIH (P=0.002). The simplified AIH score was 3.7±0.9 in the DILI group and 6.5±0.9 in the AIH group (P<0.001).
Conclusions
Accurate diagnosis is necessary for patients with a history of medication and visits for liver-function abnormalities; in particular, the possibility of AIH should be considered.
INTRODUCTION
Drug-induced liver injury (DILI) is a serious health problem with broad implications for patients, healthcare providers, the pharmaceutical industry, and governmental regulatory agencies, as it is the most common cause of withdrawal of drugs from the pharmaceutical market.1 DILI is a significant cause of morbidity and mortality, accounting for at least 13% of acute liver failure cases in the US.2
The incidence of DILI has been reported to be 1/10,000 to 1/100,000 treated patients.3 In Korea, the overall incidence of DILI in the general population is largely unknown. A multicenter pilot study found that the incidence of DILI leading to tertiary hospital admission was 24.3 persons/1000 beds.4 Also, Kang et al reported 159 cases in 5 years and Kim et al 68 cases in 7 years in retrospective single center studies.5,6 However, the actual incidence is probably higher due in part to the difficulty of diagnosis.
Furthermore, DILI is commonly misdiagnosed. An expert review of suspected DILI reports from primary and secondary care clinicians to the UK Committee on the Safety of Medicines revealed that approximately half of the cases were not DILI, and that the misdiagnoses led to a delay in arriving at the correct diagnosis, possibly affecting patient care.7
The precise and early diagnosis of DILI, including the identification of the offending drug, is clearly of importance. Because no specific markers or tests for DILI have been established, the diagnosis is usually based on circumstantial evidence.8 It relies on a great deal of speculation by the clinician, the collection of a detailed pharmacological history, the establishment of a consistent relationship between drug intake and the onset of the clinical picture, and the exclusion of alternative causes.8 This complex process of diagnosis usually requires an experienced clinician who is aware of the critical components to be weighed for an accurate diagnosis.
Clinical diagnosis of autoimmune hepatitis (AIH) and DILI are challenging, as both conditions have heterogeneous disease manifestations clinically as well as histopathologically.9 These conditions are mediated by immunological reactions and thus show considerable resemblance in clinical and histopathologic features (e.g., autoantibodies, plasma cells and eosinophils).9
The aim of this study was to describe the clinical features observed in patients with a history of medication, liver function test abnormalities as the chief complaints, and in whom liver biopsy was conducted, focusing particularly on accompaniment by AIH.
PATIENTS AND METHODS
Patients
We searched the diagnostic medical index at Soonchunhyang University Hospital for diagnoses of DILI between January 2006 and September 2007. The search was based on the presence of DILI in the text, and retrieved the medical charts of all patients with a mention of DILI, e.g., as the primary or differential diagnosis.
The medical charts of 29 patients with a history of taking medication and visits for liver function test abnormalities, and in whom liver biopsy was conducted, were evaluated.
Liver biopsy was performed in one of the following situations: (1) when the time to onset of DILI, typically measured from the first day on which the suspected agent was taken to the day of onset of symptoms or laboratory test abnormalities, was obscure; (2) when withdrawal of the suspect medication was not followed by clinical improvement; and (3) when other liver injury diagnoses could not be excluded.
Patients positive for any marker of viral hepatitis (hepatitis A, B, C viruses) or with an excessive alcohol intake, defined as consuming an average of more than 2 drinks per day for men and more than 1 drink per day for women,10 were excluded. Histologic features were evaluated. Interface hepatitis, lymphocytic/lymphoplasmocytic infiltrates in portal tracts and extending into the lobule, and hepatic rosette formation were regarded as typical for diagnosis of AIH, according to the histologic characterization presented by Hennes et al.11 Clinical, serologic, and histologic findings were compared and analyzed. The final diagnosis of liver injury was made by experienced hepatologists based on clinical judgement; the presence or absence of autoantibodies and/or gamma globulins with compatible histology and exclusion of competing etiologies. The new simplified AIH score was calculated.11
The study was approved by the Soonchunhyang Institutional Review Board, and written informed consent for participation in medical research was obtained from all patients.
Statistical analysis
Data are reported as means±standard deviations (SDs) for continuous variables or proportion of patients with a condition. Between-group differences were assessed using the Mann-Whitney test for continuous variables and Fisher's exact test for categorical variables. For all statistical analyses, we used the SPSS software (ver. 17.0; SPSS Inc., Chicago, IL, USA). A value of P<0.05 was considered statistically significant.
RESULTS
Patient characteristics
A total of 29 patients with a history of medication, liver function test abnormalities, and in whom liver biopsy was performed were identified. Selected demographic and clinical characteristics of the study population are shown in Table 1.
The mean age of the patients was 46.2±12.8 years. Eight were male and 21 (72.4%) were female. The most common symptom was jaundice (17/29; 58.6%). Three patients had fatigue, three had digestive symptoms (i.e., abdominal pain, dyspepsia, appetite loss), and six had other symptoms (i.e., fever, myalgia, headache, dizziness).
Drugs taken by the patients were herbal medications (n=16; 55.2%), prescription medications (n=9; 31.0%), and traditional therapeutic preparations and dietary supplements (n=4; 13.8%). Among subjects in whom prescription medications were implicated, the major classes of agents were antimicrobials (n=2), antihistamine agents (n=3), lipid-lowering agents (n=2), and H2-blocking agents (n=1). The implicated traditional preparations and dietary supplements were red ginseng, kudzu, yam, and chlorella.
At their first visit for liver injury events, serum biochemistry values (mean±SD) were: aspartate aminotransferase (AST), 662.2±574.8 U/L; alanine aminotransferase (ALT), 905.4±794.9 U/L; total bilirubin, 12.9±10.8 mg/dL; alkaline phosphatase (ALP), 195.8±123.3 U/L; and γ-glutamyl transpeptidase (γ-GTP), 255.3±280.8 U/L.
Comparison of clinical, serologic, and histologic features of DILI and AIH
The final diagnosis of liver injury was made by experienced hepatologists based on clinical information, the presence or absence of autoantibodies and/or gamma globulins, and histologic findings. The final diagnosis was DILI in 21 cases and AIH in eight cases.
A comparison of the results of DILI and AIH is shown in Table 2. ALT, total bilirubin, ALP, γ-GTP, and albumin levels did not differ significantly between patients with DILI and those with AIH. However, AST levels (537.1±519.1 vs. 1043.3±600.5 U/L), globulin levels (2.7±0.4 vs. 3.3±0.5 g/dL), and prothrombin time (PT; 12.9±2.4 vs. 15.2±3.9 s) were significantly higher in the AIH group (all P<0.05).
Comparison of the demographics, seropositivity, AIH score, and liver tests at presentation in patient with DILI and AIH
Antinuclear antibody (ANA) was positive in 7/21 patients with DILI and all eight patients with AIH (P=0.002). In addition, 14/21 patients with DILI showed negative ANA, three had a titer <1:80, and four had a titer ≥1:80. All patients with AIH had an ANA titer ≥1:80 (Fig. 1).

Difference in antinuclear antibody (ANA) titer between DILI and AIH. Fourteen of the 21 DILI patients were negative for ANA; 3 patients had a titer <1:80 and 4 patients had a titer >1:80. All of the AIH patients possessed a high ANA titer of >1:80.
Mean simplified AIH scores were 3.7±0.9 in the DILI group and 6.5±0.9 in the AIH group (P<0.001).
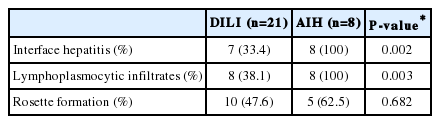
A comparison of the histologic features of patients with DILI and AIH is shown in Table 3. Interface hepatitis and lymphoplasmocytic infiltrates were present in all AIH cases, but in only one-third of DILI cases (P<0.05). Rosette formation was more frequent in AIH, but the difference was not statistically significant (P=0.682).
DISCUSSION
DILI is increasingly recognized as a cause of clinically significant acute and chronic liver disease. It is the leading cause of acute liver failure in Korea and several Western countries, and the most common reason for regulatory action regarding approved medications.2,12 Most cases of DILI are unpredictable and generally believed to be due to an immunoallergic reaction or an abnormality in the metabolism of the agent and lack a dose relationship, although a dose threshold has recently been suggested.13-15 The clinical presentation of DILI covers a wide spectrum from asymptomatic liver test abnormalities to symptomatic acute liver disease, prolonged jaundice, and disability, or overt acute or subacute liver failure.16 The recognition and diagnosis of DILI are often difficult and delayed due to the need to exclude more common causes of liver injury.16
Our data suggest that AIH comprises a significant proportion of consecutive patients with suspected DILI. That is, among the patient with a history of taking medication and a visit to the hospital for liver function test abnormalities, AIH can be unmasked in a significant number of that. Both DILI and AIH are mediated by immunologic reactions and thus show considerable similarity in their clinical and histopathologic features.9 Further, differentiation between idiopathic AIH and AIH triggered by drugs is often difficult in a clinical setting. Because it is practically impossible to exclude potential drug involvement in some cases, the differential diagnosis of DILI versus AIH can be extremely difficult.9
In this study, we compared several biochemical parameters between the DILI and AIH groups. Markers of hepatocellular liver injury (e.g., AST, ALT, PT) were higher in the AIH than the DILI group, and markers of cholestatic liver injury (e.g., ALP, γ-GTP) were higher in the DILI than the AIH group. However, most were not significantly different, except for AST and PT. Therefore, abnormalities of biochemical liver tests can aid in the diagnosis of DILI, but their utility in differentiating DILI from AIH is uncertain. Globulin level was significantly higher in the AIH group, as we expected due to the elevated immunoglobulin level in this autoimmune condition.
ANA was positive in 7/21 patients with DILI and all eight patients with AIH (P=0.002). In addition, 14/21 patients with DILI showed negative ANA, three had a titer <1:80, and four had a titer ≥1:80. All patients with AIH had an ANA titer ≥1:80. The diagnosis of AIH was considered probable if the score was more than 6 points and definite if the score was more than 7 points according to Hennes et al.11 Mean simplified AIH scores was 6.5 in AIH and 3.7 in DILI group. Therefore, our data suggest that ANA and simplified AIH score can be useful diagnostic tools for AIH.
Liver biopsy has been considered an important and at times essential element in the diagnosis of DILI.17 Nevertheless, the role of liver histology in the diagnosis and causality assessment of DILI remains unclear.12,16 Although AIH exhibits some typical histologic patterns of injury, DILI can mimic any non-DILI pattern of injury.9 Although some histologic features, such as prominence of eosinophils, granulomas, zonal or massive necrosis, and cholestasis with hepatitis, may increase the index of suspicion of DILI, no unique histologic features can unequivocally confirm the diagnosis.18 In this study, we found some histologic features with DILI cases, such as portal tract expansion by infiltration of mononuclear cells and eosinophils, centrizonal cholestasis, and focal necrosis. Clearly, objective and prospective studies of the role of liver histology in improving the diagnosis and management of DILI are necessary.
The characteristic histologic features of AIH are well-documented in the literature.19 Interface hepatitis, lymphocytic/lymphoplasmacytic infiltrates in portal tracts extending into the lobule, emperipolesis, and hepatocyte rosette formation are considered common findings of AIH, and are used in the recently published simplified criteria for its diagnosis.11 This study showed that interface hepatitis and lymphoplasmocytic infiltrates were present in all cases of AIH, but in only one-third of DILI cases. Rosette formation was more frequent in AIH, but this difference was not statistically significant.
The lack of objective confirmatory diagnostic tests coupled with the highly variable clinical presentation of DILI often leads to delayed recognition.17 Causality assessment instruments have been developed to help standardize the diagnosis. In 2003, the National Institute of Diabetes and Digestive and Kidney Diseases established the Drug-Induced Liver Injury Network (DILIN) as a long-term initiative to promote basic and clinical research involving DILI.17 Although DILI is largely a diagnosis of exclusion, DILIN has provided a framework for establishing a diagnosis of DILI that includes key clinical features.17 Going forward, we hope that standardized terminology, definitions, and minimal diagnostic elements will be included in reports of suspected DILI cases to facilitate future research.17,20
Our results suggest that accurate diagnosis is necessary for patients with a history of medication and visits for liver function abnormalities; in particular, the possibility of AIH should be considered.
Notes
The authors have no conflicts to disclose.
Abbreviations
AIH
autoimmune hepatitis
ALP
alkaline phosphatase
ALT
alanine aminotransferase
ANA
antinuclear antibody
AST
aspartate aminotransferase
DILI
drug-induced liver injury
γ-GTP
γ-glutamyl transpeptidase
PT
prothrombin time