Sarcopenia and blood myokine levels as prognostic biomarkers in patients with liver cirrhosis or hepatocellular carcinoma
Article information
Sarcopenia is defined as the progressive and generalized loss of skeletal muscle mass, strength, and function [1,2]. It primarily occurs in relation to the normal aging process, but is also described with various medical illnesses, including liver cirrhosis (LC) and hepatocellular carcinoma (HCC) [1-3]. Sarcopenia in patients with LC is classified as secondary sarcopenia associated with disease (cirrhosis), low physical activity (e.g., disuse), and/or malnutrition (e.g., protein deficiency) [1,4]. Sarcopenia is a common feature of malnutrition among patients with LC or HCC, and has been widely recognized as an independent predictor of clinical outcomes in patients with LC and as a prognostic factor in patients with HCC [1,5-8].
In the current issue of Clinical and Molecular Hepatology, Choi and colleagues [9] presented a study demonstrating serum levels of three myokines (myostatin, follistatin, and interleukin-6 [IL-6]) and their correlation with sarcopenia and survival in HCC patients. This article is timely, and it also covers critical topics on sarcopenia and its impact on survival in patients with HCC. The strength of this study relies on the novel approach used to identify the predictive biomarker of sarcopenia and survival in patients with HCC by using serum myokine levels. The authors evaluated sarcopenia using the psoas muscle index (PMI) measured at the third lumbar level on computed tomography, and reported an overall sarcopenia prevalence of 56.4% in 238 ethnically homogenous South Korean patients with HCC [9].
Myokines are cytokines produced and secreted by muscle fibers, and they are known to exert autocrine or paracrine effect [10]. Myokines take part in immune responses, and have anti-inflammatory or immunoprotective effects [11]. Therefore, sarcopenia may facilitate the proinflammatory state of cirrhosis and further potentiate the progression of liver fibrosis and development of HCC [1,12]. In the present study, Choi et al. [9] reported that the serum levels of the three myokines were differently correlated with PMI in patients with HCC. The median levels of the three myokines in the patients with HCC were all significantly higher than those in healthy controls, and the serum follistatin level was an independent factor of poor survival in the patients with HCC [9]. In a recent Japanese study, Nishikawa et al. [13] found that higher serum myostatin levels were correlated with sarcopenia, hyperammonemia, and impaired protein synthesis, as reflected by the lower serum albumin levels in patients with LC. They suggested the use of serum myostatin level as a potential biomarker, and demonstrated the association of high myostatin levels with both sarcopenia and worse survival in patients with LC [13]. In contrast, the report by Choi et al. [9] indicated an inverse correlation between serum myostatin level and sarcopenia in patients with HCC. In their study, serum myostatin levels showed a positive correlation with PMI (ρ=0.356, P<0.001), and the overall survival rate was not significantly different between the high and low myostatin groups [9]. In contrast, the serum IL-6 level showed a weak negative correlation with PMI (ρ=-0.174, P=0.009), and serum follistatin level approached statistical significance towards a negative correlation (ρ=-0.124, P=0.055). Moreover, HCC patients with high levels of follistatin or IL-6 had a significantly lower 5-year overall survival rate [9].
Myostatin is a cytokine belonging to the transforming growth factor beta (TGF-β) family. As a negative regulator of muscle protein synthesis, it strongly suppresses skeletal muscle growth [1,14]. Hyperammonemia, as a possible mediator in the liver-muscle axis, and the related upregulation of myostatin are regarded as mechanisms of the impaired protein synthesis and increased autophagy, which is linked to the development of sarcopenia in LC patients [13,15]. Protein synthesis is biochemically upregulated by the mammalian target of rapamycin complex 1 (mTORC1), which is counterbalanced by an inhibitor, myostatin (Fig. 1) [1,16]. Increased serum myostatin expression level in patients with LC is believed to be associated with anabolic resistance, and can represent an adverse predictor of patients with LC [13,17]. In view of the results from the study by Nishikawa et al. [13], serum myostatin levels appeared to be closely related to the hepatic functional reserve. In their study, serum myostatin level was significantly correlated with serum ammonia level, and this result can support the hypothesis that skeletal muscle mass depletion can occur via impaired ammonia detoxification and its related higher myostatin expression [13,18]. However, in the study by Choi et al. [9], serum myostatin level showed a positive correlation with PMI and had no significant association with the overall survival in South Korean patients with HCC. This discrepancy may have resulted from the difference in the study population, LC vs. HCC patients. Although the exact mechanism is yet to be defined, the authors suspect that the development of HCC might change the regulatory pathway of myostatin metabolism, which leads to decreased bioavailability of myostatin [9]. In addition, in the study of patients with LC by Nishikawa et al. [13], serum myostatin level did not influence the survival of patients with hepatitis C virus (HCV)-related LC. However, in the subjects with non-viral hepatitis-related LC, the influence of serum myostatin level on survival was prominent [13]. This difference might be attributed to the different causes of death for the subjects with HCV-related and non-virus-related LC. Most of the patients with non-virus-related LC died from liver failure, which was in line with the association between serum myostatin level and liver functional reserve. However, the patients with HCV-related LC or HCC tended to die from tumor progression rather than from liver failure, as compared to the patients with non-virus-related LC [13]. Choi et al. [9] also revealed that serum follistatin and IL-6 levels remarkably increased according to tumor progression by Barcelona Clinic Liver Cancer (BCLC) or tumor, nodes, metastasis (TNM) tumor stage in patients with HCC. Meanwhile, serum myostatin level did not correlate well with the TNM or BCLC tumor stage, but correlated well with the Child-Pugh class or liver functional reserve [9].
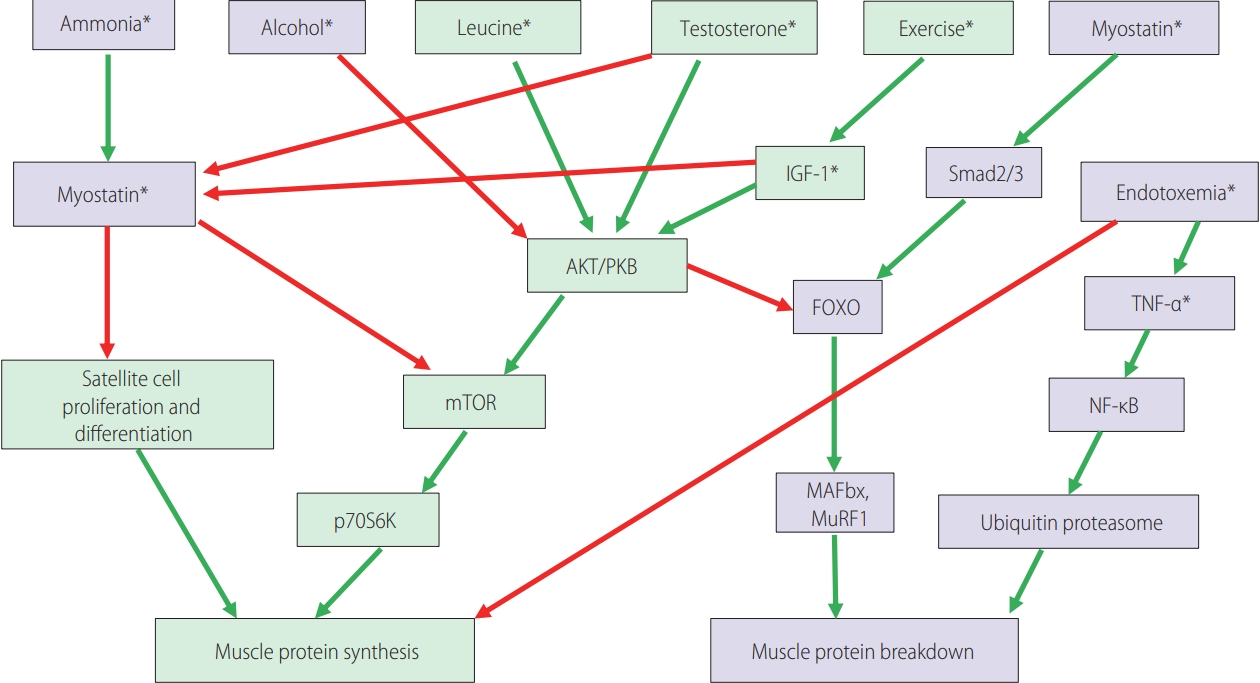
The signaling pathways involved in the control of muscle protein synthesis and breakdown. Green boxes contain promoters of muscle protein synthesis or hypertrophy. Purple boxes contain promoters of muscle protein breakdown or atrophy. Green arrows: activating pathways; red arrows: inhibitory pathways. Asterisks (*) indicate potential therapeutic targets for reversing sarcopenia in cirrhosis. Modified from Anand [1]. IGF-1, insulin-like growth factor 1; Smad2/3, SMAD family member 2/3; AKT/PKB, serine/threonine-specific protein kinase B; FOXO, forkhead transcription factors of the O class; TNF-α, tumor necrosis factor-α; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; p70S6K, ribosomal protein S6 kinase B1; MAFbx, muscle atrophy F-box; MuRF1, muscle ring finger 1.
Myostatin antagonists, such as follistatin, and mTORC1 activators have the potential to reverse sarcopenia in cirrhosis [13]. However, the influence of such agents on clinical outcomes has not been fully studied yet, and additional investigations are needed [14,19]. Follistatin is a secreted glycoprotein and a strong inhibitor of the TGF-β superfamily, which includes myostatin and activin [9,20]. Thus, follistatin inhibits myostatin-mediated sarcopenia and has been proven in animal studies to improve skeletal muscle mass, but it should not yet be used for patients with LC or HCC-related sarcopenia [1]. Nevertheless, the follistatin expression level was increased in approximately 60% of tumor tissues and also in human liver cancer [20]. This was consistent with the results reported by Choi et al. [9], where the follistatin level increased with the tumor stage and size, suggesting an oncogenic role of follistatin overexpression in hepatocarcinogenesis. Future studies are needed to address and clarify the roles of these myokines (e.g., myostatin, activin, and follistatin) during liver fibrosis and carcinogenesis in LC and HCC experimental models. In addition, several novel studies using antioxidants, mitochondrial protective agents, and new myostatin inhibitors for sarcopenic patients with LC or HCC are now being recognized [1]. Further studies are required to confirm whether antisarcopenia treatment prolongs the survival and reverses the molecular abnormalities underlying sarcopenia in patients with LC and HCC [3].
Notes
Authors’ contributions
S Oh: manuscript writing and critical revision; J Lee: critical revision and supervision
Conflicts of Interest
The authors have no conflicts of interests to disclose.
Abbreviations
BCLC
Barcelona Clinic Liver Cancer
HCC
hepatocellular carcinoma
HCV
hepatitis C virus
IL-6
interleukin-6
LC
liver cirrhosis
mTORC1
mammalian target of rapamycin complex 1
PMI
psoas muscle index
TGF-β
transforming growth factor beta
TNM
tumor, nodes, metastasis
