Efficacy and safety of metronomic chemotherapy for patients with advanced primary hepatocellular carcinoma with major portal vein tumor thrombosis
Article information
Abstract
Background/Aims
Low-dose metronomic chemotherapy involves the frequent administration of comparatively low doses of cytotoxic agents with no extended breaks, and it may be as efficient as and less toxic than the conventional maximum tolerated dose therapy. This study evaluated the feasibility and therapeutic efficacy of metronomic chemotherapy in patients with advanced hepatocellular carcinoma (HCC) with major portal vein thrombosis (PVT).
Methods
Thirty consecutive HCC patients with major PVT with or without extrahepatic metastasis were prospectively allocated to metronomic chemotherapy consisting of epirubicin being infused through the correct hepatic artery at a dose of 30 mg/body surface area (BSA) every 4 weeks, and cisplatin (15 mg/BSA) and 5-fluorouracil (50 mg/BSA) every week for 3 weeks, with intervening 1 week breaks. The treatment response was assessed using response evaluation criteria in solid tumors (RECIST).
Results
In total, 116 cycles of metronomic chemotherapy were administered to the 30 patients, with a median of 3 cycles given to individual patients (range, 1-15 cycles). Six patients (20.0%) achieved a partial response and six patients (20.0%) had stable disease. The median time to disease progression and overall survival were 63 days (range, 26-631 days) and 162 days (95% confidence interval; range, 62-262 days), respectively. Overall survival was significantly associated with baseline alpha-fetoprotein level (P=0.001) and tumor response (P=0.005). The baseline alpha-fetoprotein level was significantly associated with the disease control rate (P=0.007). Adverse events were tolerable and managed successfully with conservative treatment.
Conclusions
Metronomic chemotherapy may be a safe and useful palliative treatment in HCC patients with major PVT.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of death from cancer worldwide.1 Currently, surgical resection or liver transplantation is the curative treatment for HCC. However, only a minority of patients with HCC are eligible for these treatments because most have advanced disease at their first diagnosis and/or underlying cirrhosis. Chemotherapy including systemic chemotherapy, hepatic artery infusion chemotherapy (HAIC), and transcatheter arterial chemoembolization (TACE) was commonly used for palliative treatment for advanced HCC.
Conventional chemotherapy is based on maximum tolerated dose (MTD) chemotherapy, which means use of a single dose or a short course of the highest dose tolerable whose toxicity is not life-threatening. MTD chemotherapy mainly targets proliferating tumor cells.2,3 However, with repeated MTD chemotherapy, HCC cells can readily acquire resistance to the chemotherapeutic agent and undesirable side effects normally associated with conventional cytotoxic chemotherapy are often observed. Patients with HCC tend to experience more severe toxicities related to chemotherapy compared to patients with other cancers, due to underlying liver cirrhosis. Therefore, systemic chemotherapy using this regimen has been reported to be unsuccessful in HCC.4 As a result, there is growing need for new chemotherapeutic strategy in patients with HCC.
Metronomic chemotherapy involves the administration of a chemotherapeutic drug at a reduced dose compared with conventional MTD chemotherapy, but at regular and more frequent intervals, with no long intervening rest periods.5 Recent preclinical studies in various cancer models have demonstrated the anti-tumor and anti-angiogenesis efficacy of metronomic chemotherapy.6-8 Our previous study additionally showed that metronomic chemotherapy scheduling is at least as efficient as MTD therapy in suppressing the growth of HCC tumors, and is less toxic in a HCC model with associated liver cirrhosis. Furthermore, an antiangiogenic effect was evident and manifest as inhibition of tumor endothelial cells.9
Despite the implied potential value of metronomic chemotherapy, it has been scantly utilized in patients with HCC. In this pilot study, the feasibility and therapeutic efficacy of metronomic chemotherapy using conventional chemotherapeutic agents was evaluated in patients with advanced HCC with portal vein invasion.
PATIENTS AND METHODS
Patients
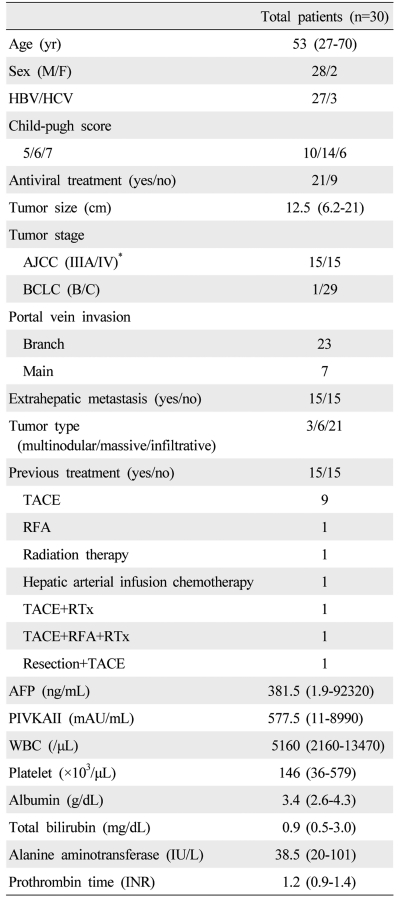
Between June 2005 and June 2009, 30 patients with intractable and advanced HCC were recruited to this pilot trial of low dose metronomic chemotherapy. Intractable and advanced HCC was defined as HCC with portal vein invasion, infiltrative type and/or refractory to surgical resection or nonsurgical intervention (transcatheter arterial chemoembolization, radiofrequency ablation and percutaneous ethanol injection). A diagnosis of HCC was based on either pathologic confirmation or radiologic findings with an elevated level of serum alpha-fetoprotein (AFP) (>400 ng/mL) in the setting of liver cirrhosis. Initially, for inclusion into the study, patients eligible patients met the following criteria: >18-years-of-age, Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, adequate liver function defined as Child-Pugh score of ≤7, acceptable blood cell counts (absolute neutrophil count of ≥1,500 cells/mm3, platelet count ≥60,000 cells/mm3, white blood cell count ≥4,000 cells/mm3), serum creatinine level ≤1.5 mg/dL, and vascular accessible lesion for the implantation of a chemoport. However, because there was few treatment option for patients with advanced HCC, patient with good performance status and favorable liver function was enrolled even if they had low blood cell counts. Portal vein invasion was defined as having a tumor thrombus in the main trunk (Vp4) and the first-order branch (Vp3) of the portal vein. Patients were excluded if they had one or more of the following: evidence of hepatic decompensation including uncontrolled ascites, gastrointestinal bleeding or hepatic encephalopathy; total bilirubin level ≥3 mg/dL or prolongation of prothrombin time by more than 3 seconds; concurrent serious medical condition(s) such as underlying cardiac or renal diseases; or other concurrent primary malignancy. The trial was conducted according to the current declaration of Helsinki, and the protocol was approved by the institutional ethics committee of the Catholic University of Korea. Written informed consent was obtained from each patient.
Protocol of low dose metronomic chemotherapy
Chemotherapeutic agents were delivered through a percutaneous hepatic arterial catheter (chemoport) implanted at the time of initial hepatic arterial angiography. The catheter insertion and placement were performed as previously described.10 The metronomic chemotherapeutic agents were cisplatin and 5-fluorouracil (5-FU). Cisplatin and 5-FU was infused weekly for 3 weeks with intervening 1 week break. A single dose of cisplatin and 5-FU was 15 mg/m2 and 50 mg/m2, respectively. Along with the metronomic chemotherapy, epirubicin (30 mg/m2) was infused monthly without use of lipiodol and gelfoam embolization. This protocol was repeated every 4 weeks and continued until disease progression or unacceptable toxicity was evident, or the patient refused to continue (Fig. 1). In case of cisplatin infusion, intravenous hydration was performed to prevent nephrotoxicity and an appropriate anti-emetic agent was given to all patients. Dose adjustments were made depending on the toxicity observed in the preceding treatment cycle. The following cycle of treatment was reduced by 30% in the case of repeated grade 2 toxicity during the preceding cycle. Treatment was stopped in the case of any grade 3 or grade 4 toxicity until resolution. If a patient required a delay of >4 weeks for recovery, they were excluded from the study.
Study assessments
The primary endpoint of the current study was feasibility. Feasibility was assessed by the proportion of patient who could undergo at least two complete sessions of metronomic chemotherapy without adverse event. The secondary endpoints were an objective response rate (complete response plus partial response [PR]), time to disease progression and overall survival. Tumor response was evaluated using abdominal dynamic computed tomography after every two complete sessions of chemotherapy. The responses were evaluated using Response Evaluation Criteria In Solid Tumors criteria on an intention-to-treat basis.11 The tumor responses were categorized as CR, PR, stable disease (SD), or progressive disease (PD). On computed tomography scans obtained after treatment, the sums of the longest diameter for all target HCCs was measured. Complete response was defined as the disappearance of any intratumoral arterial enhancement in all target lesions. PR was defined as at least a 30% decrease in the sum of the longest diameter of viable (contrast enhancement in the arterial phase) target lesions taking as reference the baseline sum longest diameter. PD was defined as at least a 20% increase in the sum of the longest diameter of viable (enhancing) target lesions or the appearance of one or more new lesions. SD was defined as a tumor status that did not meet the above three response criteria. For the evaluation of endpoint, the best response for each patient was selected. Liver function, renal function, complete blood counts, serum AFP, and chest X-ray were also evaluated at each visit. Toxicity was scored using the Common Terminology Criteria for Adverse Events (CTCAE; version 3.0)12 at every visit. Survival and time to disease progression was estimated from the date of treatment initiation.
Statistical analyses
Continuous data were expressed as medians and ranges, and categorical data as percentages. Comparison between groups was performed using the Chi-square and/or Fisher's exact tests, and Mann-Whitney U-test, as appropriate. Cumulative survival and time to disease progression rates were calculated using the Kaplan-Meier method and the difference between groups were compared by the log-rank test. A Cox proportional-hazards model was used to reveal independent clinical factors or groups affecting response rate and overall survival. Factors less than 0.1 in univariate analysis was further analyzed as cofactor in multivariate analysis. All data was analyzed using SPSS version 14.0 software (SPSS, Chicago, IL, USA).
RESULTS
Patient characteristics
Thirty patients were enrolled into the study between June 2005 and June 2009. Table 1 summarizes the patient's baseline characteristics. The median age was 53 years and 93% of the patients were male. The underlying liver disease was predominantly hepatitis B virus infection (90%) and hepatic function was mostly Child-Pugh class A (80%). All patients had portal vein tumor thrombosis (PVT) and 12 (40%) patients had a liver tumor burden ≥50%. At enrollment, 15 patients (50%) had extrahepatic metastasis in the lung (n=9), lymph node (n=1), bone (n=1), abdominal wall (n=1), both bone and lymph node (n=2) and both bone and lung (n=1). Fifteen patients (50%) displayed progressive disease to other treatments for HCC before metronomic chemotherapy.
Feasibility
One hundred sixteen cycles of metronomic chemotherapy with a median of three cycles (mean 3.8 cycles; range, 1-15 cycles) for each patient was administered to the 30 patients. Twenty two (73%) patients received at least two complete sessions of metronomic chemotherapy, The remained 8 patients received one complete session but did not complete two session of chemotherapy due to adverse event (neutropenia, n=4, GI bleeding, n=1) and aggravated liver function due to tumor progression (n=1) or ingestion of herb (n=2). The median dose of anti-cancer agents per cycle was 58 mg (range, 35-74 mg) for epirubicin, 75 mg (range, 45-105 mg) for cisplatin, and 256.95 mg (range, 150-450 mg) for 5-FU. The cumulative median dose for each drug during the entire treatment was 162.5 mg (range, 35-960 mg) for epirubicin, 225 mg (range, 60-1215 mg) for cisplatin, and 757 mg (range, 225-4050 mg) for 5-FU. In 15 patients with extrahepatic metastasis, 11 received concurrent radiation therapy for the metastatic lesion, while the other four patients did not because metronomic chemotherapy was prematurely terminated due to adverse events or progression.
Tumor response
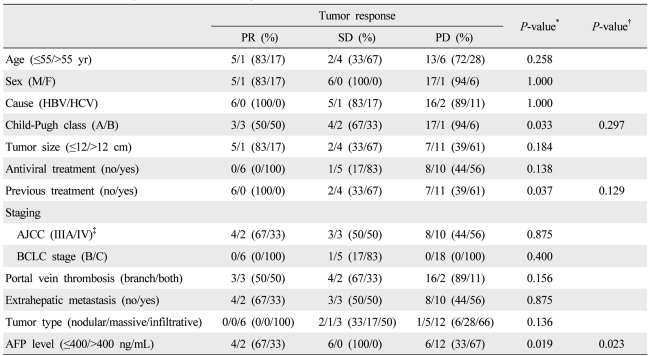
After start of metronomic chemotherapy, tumor response could be evaluated in all 30 patients at a median of 36 days (range, 22-121 days). Of 22 patients who received at least two complete sessions of metronomic chemotherapy, PR was achieved in 23% (5/22), SD was observed in 23% (5/22) and PD was noted in 54% (12/22). Of 8 patients who did not complete two session of chemotherapy, PR was achieved in 12.5% (1/8), SD was observed in 12.5% (1/8) and PD was noted in 75% (6/8). In overall, PR was achieved in 20% (6/30), SD was observed in 20% (6/30) and PD was noted in 60% (18/30). Of the 12 patients with PR or SD initially, six patients showed PD during metronomic chemotherapy at a median of 102 days (range, 72-414 days) and overall progression rate was 80% (24/30). Total duration of metronomic chemotherapy was median 78 days (range, 27-489 days). After metronomic chemotherapy, only 6 patients out of 24 patients with progression underwent further treatment and remained patient did not receive further treatment due to aggravated liver function or performance status. Meanwhile, 1 patient out of 6 patients with objective response received further treatment and other patients did not receive further treatment due to stable tumor status after the end of metronomic chemotherapy. In overall, the median time to progression was 63 days (range, 26-631 days). After the treatment, the median AFP level changed from 381 (range, 1.93-92320) to 371 (range, 1.62-439980) ng/mL. According to the treatment response, the median AFP level changed from 230.44 (range, 5.39-3955) to 18.41 (range, 6.73-2703) ng/mL in patients with PR, from 51.05 (5.15-374) to 106.57 (4.35-658) ng/mL in SD, and from 1455.50 (range, 1.93-92320) to 2988.00 (range, 1.62-439980) ng/ml in PD. Of the various baseline factors, history of previous treatment for HCC, baseline Child-Pugh class, and baseline serum AFP level were significantly associated with tumor response on univariate analysis. Only baseline serum AFP level remained significant on multivariate analysis (Table 2). None of the 15 patients who experienced PD after a previous treatment showed an objective response (four SD and 11 PD) while six of the 15 patients (40%) who received metronomic treatment as the initial HCC therapy displayed PR (P=0.023). Tumor response was not affected by the presence of extrahepatic metastasis. Five patients (33%) with extrahepatic metastatsis showed PR or SD. Figure 2 depicts a sample case with extrahepatic metastasis at baseline, in which marked tumor shrinkage was evident on a follow-up computed tomography scan following three cycles of metronomic chemotherapy. Blood samples were obtained from this HCC patient with lung metastasis by venipuncture before each weekly chemotherapy. Solid phase-enzyme linked immunosorbent assay (ELISA) was used to determine the serum values of VEGF (R & D Systems, Minneapolis, MN, USA).
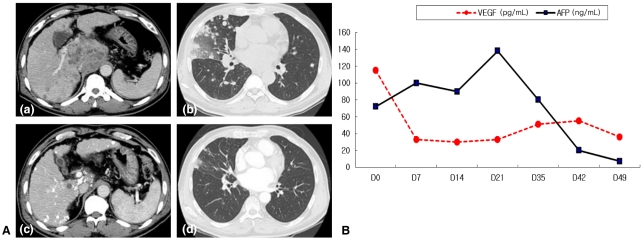
(A) An example case: a 54-year-old man was diagnosed with advanced hepatocellular carcinoma (HCC) with lung metastasis and treated with metronomic chemotherapy; (a) and (b) are initial abdominal computed tomography scans, which showed a mass in the caudate lobe (a) and multiple hematogenous metastatic nodules in both lungs (b). After three cycles of metronomic chemotherapy, the size of the tumor (c) and pulmonary metastatic lesions (d) had decreased markedly on follow-up computed tomography. (B) Serum vascular endothelial growth factor (VEGF) and alpha-fetoprotein (AFP) levels before and after metronomic chemotherapy in this sample case. VEGF decreased rapidly even after the first chemoinfusion, while the AFP response appeared after the completion of only one cycle of metronomic chemotherapy.
Survival and prognostic factors
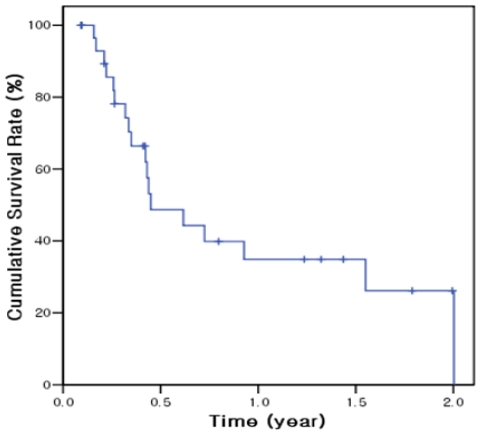
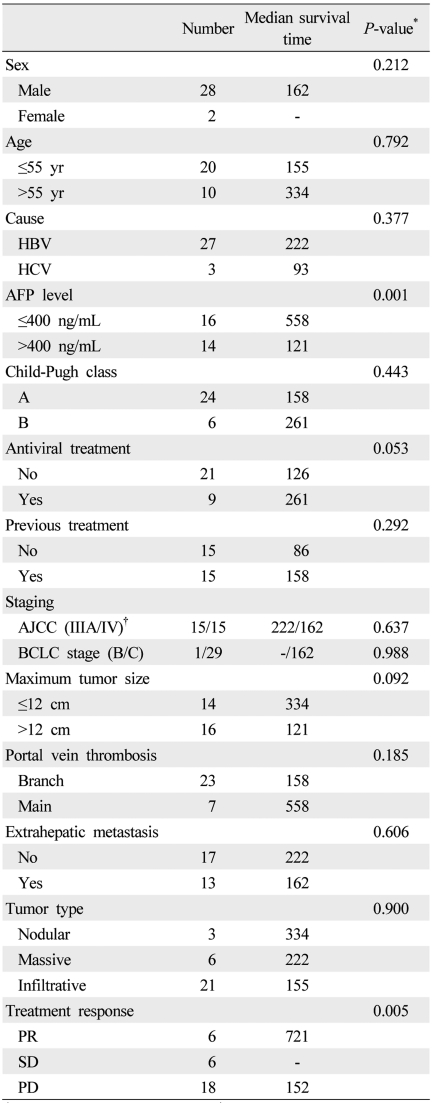
The total follow-up time after start of metronomic chemotherapy was median 151 days (mean 239 days; range, 33-721 days) and the follow-up period after the end of metronomic chemotherapy was 60 days (mean 131 days; range, 6-605 days). At the end of follow-up, 12 of 30 (40%) patients were still alive. The median overall survival was 162 days (95% confidence interval 62-262 days) and 6-month and 1-year cumulative survival rates were 48.7% and 34.8%, respectively (Fig. 3). On univariate analysis, baseline serum AFP level and tumor response were significantly associated with survival rate (Table 3). Overall survival was significantly longer in patients with baseline AFP ≤400 ng/mL (558 days) than at >400 ng/mL (121 days; P<0.001). However, in multivariate analysis, no factor was found to be significantly associated with survival probably due to small number of enrolled patients.
Adverse events
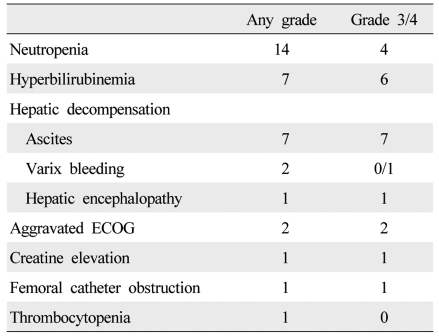
During treatment, 36 adverse events occurred, and dose reduction or treatment delay was necessary in 21 patients (Table 4). The causes of dose reduction or treatment delay were neutropenia (n=14), aminotransferase elevation (n=1), aggravated ECOG score (n=2), creatine elevation (n=1), hyperbilirubinemia (n=2) and varix bleeding (n=1). Of these, one patient with aggravated ECOG score and one patient with varix bleeding started metronomic chemotherapy with a reduced dose. Most cases of neutropenia were manageable with conservative care and granulocyte colony-stimulating factor injection, while four patients with neutropenic fever had to permanently discontinue the treatment due to PD (n=3) and concern with recurrent neutropenia (n=1), and were managed with broad spectrum antibiotics. Meanwhile, only one of the 30 patients continued the treatment at the end of follow-up. Others stopped the treatment permanently due to tumor progression (n=7), hepatic failure associated with tumor progression (n=11), hyperbilirubinemia (n=2), noncompliance (n=4), femoral catheter dysfunction (n=1), aggravated ECOG score (n=2), varix bleeding (n=1), and neutropenia (n=1).
DISCUSSION
In preclinical models, metronomic chemotherapy has several advantages in the treatment of cancer. First, it can be effective in treating tumors in which the cancer cells have developed resistance to the chemotherapeutic agent. This is because its target is not the drug-resistant cancer cell population but the drug-sensitive tumor endothelium; impressive anti-angiogenic and anti-tumor effects have been documented.3,5,13 Secondly, because the cumulative dose with metronomic therapy is significantly less than with MTD-based chemotherapy,14,15 it might be less acutely toxic, making more prolonged treatments possible and lessening or even removing the need for growth factor support to accelerate recovery from myelosuppression. Several clinical trials using metronomic chemotherapy have been reported or are underway for various type of malignancies.16,17
In HCC, metronomic chemotherapy has rarely been tried because systemic chemotherapy using conventional cytotoxic agents has not been effective in HCC therapy.4 To our knowledge, this study is the first report about metronomic chemotherapy in patients with HCC. About feasibility with this treatment, about 70% of enrolled patients could undergo at least two complete sessions of metronomic chemotherapy without adverse event. However, treatment response could be evaluated in all patients including patients with single sessions of metronomic chemotherapy. For the response, the objective response rate and median survival duration of the 30 patients were 20% and 162 days, respectively. Although there was no control group in this study, the dropout rate is slightly higher and the response rate and overall survival are slightly shorter than that of previous reports about HAIC.10,18,19 It is probably because this study included not only patients with advanced stage and resistant to other HCC treatments, but also some patients with poor liver function and low blood cell count. About 27% of the patients resistant to previous HCC treatments, 33.3% of patients with extrahepatic metastasis and 83.3% of patients with Child-Pugh class B at baseline showed PR or SD.
In this study, metronomic dose scheduling using conventional chemotherapeutic agents was delivered to 30 patients with advanced HCC with PVT via a hepatic artery infusion system. In general, the tumor cells in HCC receive their blood supply mainly from the hepatic artery, which supports the basic concept of transarterial chemotherapy. Using HAIC, chemotherapeutic agents can be delivered to the liver at a high concentration and lower toxicity.19 Several HAIC treatments using different types of chemotherapeutic regimen and treatment schedule have been tried to treat HCC. The chemotherapeutic regimen used in this study was modified based on our previous reports about the efficacy of combination therapy of transcatheter arterial infusion of and systemic infusion of 5-FU for unresectable HCC.20,21 In these studies, the efficacy of the epirubicin, cisplatin and 5-FU regimen was superior to that of conventional adriamycin treatment, although there was a lack of randomization. In the present study, the total dose of cisplatin and 5-FU was less than 75% of previous study, and one-third of the total dose was delivered weekly. Meanwhile, epirubicin infusion was performed monthly following a previously established protocol, in light of the absence of reports about frequent dosing HAIC using epirubicin. Therefore, the protocol in this study is mixed form of metronomic and conventional chemotherapy; additional studies are needed to find the best metronomic protocol for HCC.
With regard to adverse effects, most of the patients generally tolerated the metronomic chemotherapy well. Neutropenia was the most frequent side effect. Overall, hematologic complications occurred more frequently than we expected. In this study, the dose and schedule of epirubicin was not metronomic schedule but MTD schedule, different from 5-FU and cisplatin. This would be one of the causes of the frequent neutropenia. Meanwhile, hepatic complications were observed less frequently, although most of the patients had liver cirrhosis and portal hypertension. This was probably because there was no ischemic insult due to embolization and the total dose of chemotherapeutic agent was markedly reduced. In addition, the number of chemotherapy cycles and total duration of treatment were significantly longer than our previous HAIC study.10 This means that metronomic chemotherapy might be tolerable in patients with liver cirrhosis. However, the long term systemic effect of each chemotherapeutic agent should not be overlooked and might be a cause of neutropenia. Whether the benefits of metronomic chemotherapy outweigh the adverse consequences needs to be investigated, especially since the present study design did not include a control arm such as MTD chemotherapy.
Among the various pretreatment factors, only serum AFP level was significantly associated with improved patient survival. Moreover, patients with a serum AFP level ≤400 ng/mL had a better tumor response than those with an AFP level >400 ng/mL. It is very important to know who would respond or not respond to a specific treatment before that treatment is undertaken. One study reported that patients with low serum AFP respond better to 5-FU.22 However, it is unclear whether serum AFP level could be exploited as a prognostic factor for not only 5-FU containing chemotherapy but also all kinds of metronomic chemotherapy, regardless of regimen. There was no control group (i.e., maximal tolerated dose chemotherapy) in this study. However, all included patients had main or first branch portal vein invasion. Therefore, conventional TACE using epirubicin, cisplatin and 5-FU might be inappropriate in those patients. Instead, we tried to compare the efficacy and safety of metronomic chemotherapy with historical control using HAIC. Secondly, a previous report about metronomic chemotherapy documented an anti-angiogenic effect using VEGR-2 expression in tumor or serum VEGF, TSP1 and VEGFR1 levels.9 However, because there was no stored serum or tissue in most of the present patients, the anti-angiogenic effect of metronomic chemotherapy could not be evaluated with those factors. Instead, VEGF data of one sample case was available (Fig. 2); the results suggest that VEGF could be a potential predictive marker for an anti-angiogenic effect of metronomic chemotherapy.
In conclusion, this pilot study indicates that metronomic chemotherapy using hepatic arterial infusion of cisplatin and 5-FU might be a feasible and promising treatment approach for patients with advanced HCC with portal vein invasion without other treatment options. More studies will be needed to confirm the efficacy and safety of metronomic chemotherapy for patients with advanced HCC.
Acknowledgments
This work was supported by a National R & D Program grant for cancer control, Ministry of Health, Welfare and Family Affairs, Republic of Korea (R0620390-1) and the 2011 clinical research fund of the Busan University Hospital partially.
Abbreviations
AFP
alpha-fetoprotein
BSA
body surface area
CR
complete response
ECOG
Eastern Cooperative Oncology Group
HAIC
hepatic arterial infusion chmoetherapy
HCC
hepatocellular carcinoma
MTD
maximum tolerated dose
PD
progressive disease
PR
partial response
PVT
portal vein thrombosis
RECIST
response evaluation criteria in solid tumors
SD
stable disease
TACE
transcatheter arterial chemoembolization
VEGF
vascular endothelial growth factor
5-FU
5-fluorouracil