Development and validation of a simple index system to predict nonalcoholic fatty liver disease
Article information
Abstract
Background/Aims
Abdominal ultrasonography is useful for the detection and diagnosis of nonalcoholic fatty liver disease (NAFLD). The aims of this study were to establish a predictive model for the selection of subjects for abdominal ultrasonography for the diagnosis of NAFLD and to assess validity of the model.
Methods
The subjects included 901 people who visited the health examination center of the Busan Medical Center. We conducted multiple logistic regression analyses of potential risk factors to identify independent risk factors for NAFLD, and developed an index system.
Results
Four independent risk factors were identified. The index system was developed by assigning 1 clinical scoring point to approximately 0.7 logistic regression coefficients to each factor as follows: alanine aminotransferase/aspartate aminotransferase ratio >1.5 (odds ratio [OR], 2.22; 95% confidence interval [CI], 1.21-4.07; P=0.010), 1 point; γ-glutamyl transpeptidase >50 (OR, 2.15; 95% CI, 1.13-4.07; P=0.019), 1 point; triglyceride >150 mg/dL (OR, 1.92; 95% CI, 1.14-3.24; P=0.015), 1 point; 23 kg/m2≤BMI<25 kg/m2 (OR, 3.68; 95% CI, 2.05-6.63; P<0.001), 2 points; and BMI 25 kg/m2 (OR, 7.65; 95% CI, 4.29-13.62; P<0.001), 3 points. The area under the receiver operating characteristics curve was 0.797 (95% CI, 0.751-0.842), and when 3 points was used as a cut-off value, the sensitivity and specificity were 71.7% and 75.9%, respectively.
Conclusions
NAFLD can be predicted through the clinical application of the index system established herein. If abdominal ultrasonography is used for high-risk patients, NAFLD will be diagnosed and managed in its early stage.
INTRODUCTION
Non alcoholic fatty liver disease (NAFLD) is a condition with pathological findings similar to those of alcoholic fatty liver disease in non-drinkers or patients with no history of sufficient drinking to cause damage to the liver. NAFLD presents various progression ranging from simple steatosis to non alcoholic steatohepatitis (NASH) which accompany extensive inflammation or fibrosis.1 The term NASH was first used in 1980 by Ludwig et al,2 who assembled the clinical and pathological characteristics of 20 patients with no history of significant drinking enough to cause damage to the liver. Since NAFLD has no or non-specific clinical symptoms in most cases, it is diagnosed by chance in many cases during abdominal ultrasonography.3 In the West, NAFLD is reported to be the most common liver disease, with prevalence rates in general populations at 10-24%, and it is reported that the prevalence rate reaches 74% in obese populations.4,5 Hepatocellular carcinoma can also develop from the progression of fatty liver disease.6 It is known that NAFLD can develop from various causes including drugs, obesity, type 2 diabetes, and dyslipidemia.4,7 Recently, the prevalence rate of NAFLD has been increasing in Korea as well due to changes in life style. Prevalence rate of NAFLD varies among studies but has been seen in the range of 18.6-47.3%.8,9
Recent study showed that in case of normal findings of laboratory tests and physical examination, only 4.1% of all general health examinations result in prognosis improvement and disease prevention by routine use of abdominal ultrasonography as a screening test.10 In the aspect of cost-effectiveness, it is considered desirable to use abdominal ultrasonography only when abnormalities are found in laboratory tests or physical examinations. Therefore, it is considered, if abdominal ultrasonography is used when NAFLD is predicted through laboratory tests and physical examination, NAFLD will be early diagnosed, appropriately treated, and effectively managed.
The purpose of this study was to establish a predictive model to select subjects for abdominal ultrasonography for the diagnosis of NAFLD and assess the validity of the model.
PATIENTS AND METHODS
Patients
Subjects of this study included 901 persons who visited the Busan Medical Center between July 2007 and December 2008 for health examinations. Of the total subjects, seven persons who did not undergo abdominal ultrasonography and the first examination records of 11 persons who underwent health examinations twice were excluded. Other exclusions included 66 persons who were diagnosed with liver diseases other than fatty liver disease in abdominal ultrasonography; 28 patients with positivity of HBsAg; 9 patients with positivity of anti-HCV; 203 persons with daily alcohol intakes of ≥20 g; and 121 persons with no data on alcohol intake. The remaining 456 persons (178 males and 278 females) were included in the study. Of the 456 persons included, 145 persons were diagnosed as fatty liver disease by abdominal ultrasonography, and they were assigned to a NAFLD group. The remaining 311 persons were identified with normal livers, and they were assigned to a control group.
Abdominal ultrasonography
Abdominal ultrasonography was conducted by the one radiologist in all the cases and the degree of fat deposition was divided into mild, moderate, and severe. In this study, presence of fatty liver disease was used rather than its severity.
Data collection
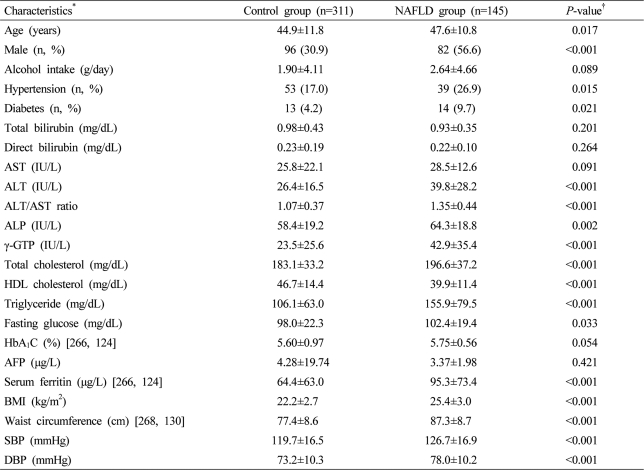
Alcohol intake was examined for the amount of drinking per time and the number of times of drinking per week through inquiries. Daily alcohol intake was calculated by converting alcohol per one bottle of soju (most popular Korean liquor) into approximately 70 g. Weights and heights were measured using an obesity-measuring device (DS-102, DSJenix Co., Ltd, Seoul, Korea) and body mass index (BMI) was calculated by dividing measured weights (kg) by the square of heights (m2). Waist circumferences were obtained by measuring the area in the halfway point between the lowest part of the ribs and the iliac crest in a standing position after a maximum expiration. Blood pressure was measured on the right upper arm using an electronic blood pressure manometer (Easy X800, Jawon Medical Co., Ltd., Gyeongsan, Korea) at a sitting position after being in a resting state for at least 10 minutes, and if the resting blood pressure of a person was 140/90 mmHg or higher, blood pressure was measured two times and the average value was recorded. Hypertension was diagnosed in patients who were taking drugs for hypertension, had been previously diagnosed with hypertension, or had blood pressure ≥140/90 mmHg. Diabetes was diagnosed in patients who were taking drugs for diabetes, had been previously diagnosed with diabetes, or had hemoglobin A1C (HbA1C) values ≥6.5%.11 Blood was collected in the morning after a 12-hour fast for biochemical tests. Total bilirubin, direct bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GTP), total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglyceride, fasting glucose, HbA1C, α-fetoprotein (AFP), serum ferritin, and ALT/AST ratio were analyzed.
Statistical analysis
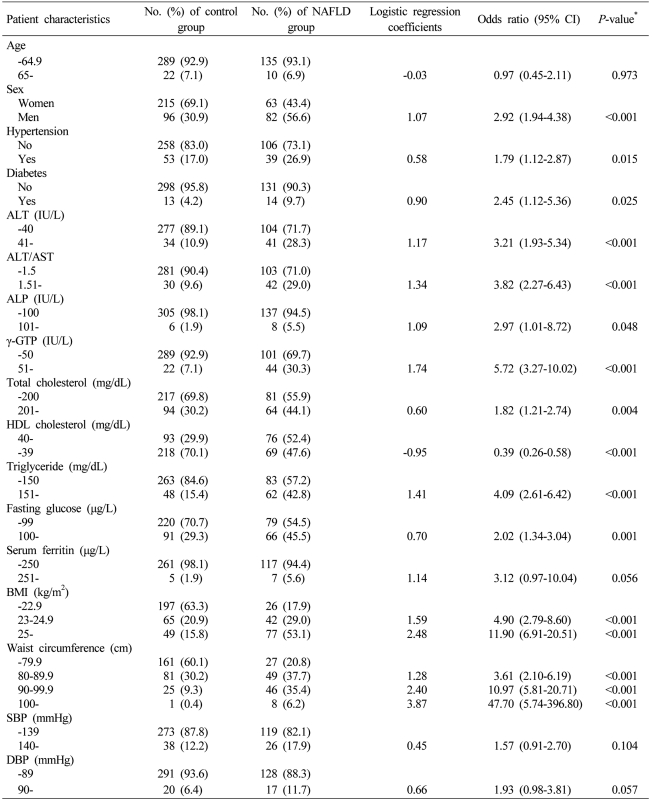
Collected data were statistically analyzed by SPSS Ver. 13 (SPSS, Inc., Chicago, USA) for Windows. The study subjects were divided into a NAFLD group and a control group. Independent sample t-tests were conducted with age, daily alcohol intake, total bilirubin, direct bilirubin, AST, ALT, ALT/AST ratio, ALP, γ-GTP, total cholesterol, HDL-C, triglyceride, fasting glucose, HbA1C, AFP, serum ferritin, BMI, waist circumference, systolic blood pressure (SBP), and diastolic blood pressure (DBP), and the values were presented as mean±standard deviation. Chi-square tests were conducted with sex, hypertension and diabetes. Simple logistic regression analyses were conducted with variables that had statistically significant differences at significance levels <0.05 in the t-tests and the chi-square tests. Multiple logistic regression analyses were conducted to identify independent risk factors of NAFLD with variables that had statistically significant differences at significance levels <0.05 in the simple logistic regression analyses. Then, we assigned clinical scores in integers to logistic regression coefficients obtained multiple logistic regression analyses, thereby induced the index system. The index system was applied to the study groups to assess the validity of the index system. All of the statistical analyses were conducted with two-tailed tests and statistically significant levels were determined as being <0.05.
RESULTS
Prevalence rate of NAFLD
Of the total 456 study subjects, 178 (39.0%) were males and 278 (61.0%) were females, and their average age was 45.8 ± 11.5 years. Since all the subjects were identified as consuming <20 g of alcohol a day, the 145 (31.8%) subjects who were diagnosed with fatty liver disease on abdominal ultrasonography were diagnosed as NAFLD. A total of 82 (46.1%) males and 63 (22.7%) females were diagnosed as NAFLD.
Clinical characteristics of the NAFLD group
The mean age of the NAFLD disease group was 47.6 years which was higher than that of the control group, which was 44.9±11.8 years (P=0.017). There were 82 (56.6%) males in the NAFLD group, which was more than that of the control group (56.6 vs. 30.9%, P<0.001). The mean BMI and waist circumference were higher in the NAFLD group than in the control group. SBP, DBP, and ratios of hypertension patients were also higher in the NAFLD group. Although the prevalence of diabetes mellitus and fasting glucose were higher in the NAFLD group, HbA1C had no significant difference between the two groups. AST had not significantly different between the two groups while ALT, ALT/AST ratio, ALP, γ-GTP, total cholesterol, triglyceride, and serum ferritin were higher in the NAFLD group, but HDL-C was lower in the NAFLD group than in the control group (Table 1).
Index system using NAFLD-related predictive factors
The relative risk factors of NAFLD were examined by conducting simple logistic regression analyses for factors that had statistically significant differences between the NAFLD group and the control group in Table 1. Except for age, serum ferritin, SBP, and DBP, all variables were significantly increased in the relative risk of NAFLD (Table 2). Multiple logistic regression analyses were conducted using the factors that had significantly increased relative risks in the simple logistic regression analyses. Based on the results, four independent risk factors (ALT/AST ratio, γ-GTP, triglyceride, BMI) of NAFLD were identified. In order to induce the index system using the results of the multiple logistic regression analyses, clinical scores in integers were given to logistic regression coefficients. By giving 1 point of clinical score to around 0.7 of logistic regression coefficients, the index system ranged from 0 point at the minimum to 6 points at the maximum. The points was given to each factor as follows: ALT/AST ratio > 1.5 (OR, 2.22; 95% CI, 1.21-4.07; P=0.010), 1 point; γ-GTP > 50 (OR, 2.15; 95% CI, 1.13-4.07; P=0.019), 1 point; triglyceride > 150 mg/dL (OR, 1.92; 95% CI, 1.14-3.24; P=0.015), 1 point; 23 kg/m2 ≤ BMI < 25 kg/m2 (OR, 3.68; 95% CI, 2.05-6.63; P<0.001), 2 points; and 25 kg/m2 ≤ BMI (OR, 7.65; 95% CI, 4.29-13.62; P<0.001), 3 points (Table 3).
Validity of the index system for NAFLD
To assess the validity of the induced index system, we applied it to the relevant study groups and obtained its ROC curve (Fig. 1), sensitivity, specificity, positive predictive value, and negative predictive value (Table 4). The AUC of the ROC curve was 0.797 (95% CI: 0.751-0.842); when 3 points were used as a cut-off value, sensitivity was 71.7% and specificity was 75.9%; when 4 points were used as a cut-off value, sensitivity was reduced to 46.9%, specificity increased to 92.3%, the positive predictive value was 73.9%, and the negative predictive value was 78.8%.

The receiver operating characteristics (ROC) curve of the index system developed for the prediction of nonalcoholic fatty liver disease. The area under the ROC curve is 0.797 (95% confidence interval, 0.751-0.842), and when 3 points is used as a cut-off value, the sensitivity and specificity are 71.7% and 75.9%, respectively.
DISCUSSION
NAFLD is one of the most common liver diseases, not only in the West but also in Korea.4,5,8,9 Prevalence rate of NAFLD is also gradually increasing. In addition, most patients with NAFLD do not know that they have the disease or live without any treatment even after diagnosis since they have no symptoms. However, since NAFLD can develop into liver cirrhosis or even hepatocellular carcinoma, it should be detected early and managed appropriately.1,6,7
The prevalence rate of NAFLD diagnosed by abdominal ultrasonography in Korea was surveyed to be 19.9% in 1994.12 The prevalence rate of NAFLD is further increased, as obese populations has been increased along with changes in life styles. Recently, prevalence rates of NAFLD is surveyed in the range of 18.6-47.3%.8,9 In this study, of the 456 study subjects, 145 (31.8%) study subjects were diagnosed as NAFLD; thus, the prevalence rate did not differ from the results of previous studies in Korea but was higher than the results of studies of NAFLD prevalence rate in the West (10-24%).4
In this study, four independent risk factors (ALT/AST ratio, γ-GTP, triglyceride, and BMI) were identified. Compared to the known risk factors of NAFLD which are obesity, diabetes, and dyslipidemia, the factors were consistent with the results of this study except for diabetes. ALT which is a finding that can indicate NAFLD in blood tests was also consistent with the result (ALT/AST ratio) of this study.4,13 In this study as well, the prevalence of diabetes was significantly different between the 2 groups (4.2% in the control group and 9.7% in the NAFLD group), but it was not an independent risk factor. This is thought to be due to the fact that the number of subjects in the study groups was small. Alcohol intake was limited to <20 g per day, but increased γ-GTP was identified as an independent risk factor of NAFLD. That can be thought that even low alcohol intake is a risk factor of fatty liver disease. However, various cross-sectional studies were presented that γ-GTP increases is associated with insulin resistance or metabolic syndrome.14,15
Harrison et al16 proposed a scoring system that was intended to diagnose and treat patients with NAFLD who also had fibrosis with at least mild degree. In that study, the scoring system ranged from 0 point to 4 points. The points was given to each factor as follows: BMI ≥28 kg/m2, 1 point; AST/ALT ratio ≥0.8, 2 points; and if diabetes mellitus, 1 point. Cases with ≥2 points was associated with an odds ratio for advanced fibrosis of 17 and a negative predictive value of 96%. However, in that study, the scoring system was focused on fibrosis rather than diagnosis of NAFLD and used liver biopsy instead of ultrasonography for diagnosis of fibrosis.
Lee et al17 derived a formula as a screening tool for NAFLD that can be utilized for selecting individuals for liver ultrasonography. The formula was derived using a logistic regression model: hepatic steatosis index (HSI) = 8×(ALT/AST ratio) + BMI (+2, if female; +2, if diabetes mellitus). At values of < 30.0 or > 36.0, HSI ruled out NAFLD with a sensitivity of 93.1%, or detected NAFLD with a specificity of 92.4%, respectively. However, that study also used ultrasonography instead of liver biopsy for diagnosis of steatosis, and the accuracy of the diagnosis in that study was similar to that in this study despite difference of variables and complex formula.
This study had several limitations. Ultrasonography rather than liver biopsy was used for diagnosis of NAFLD. Ultrasonography has lower accuracy than liver biopsy.18 In addition, the role of ultrasonography in the diagnosis of steatohepatitis has been identifying the existence and degree of steatosis, but ultrasonography is not effective in differentiating between simple steatosis and steatohepatitis.19 However, since steatohepatitic tissue is not evenly distributed across the liver parenchyma, findings from liver biopsy of a partial and small amount of the liver can not be represented of the entire liver.20 On the other hand, ultrasonography has high sensitivity, and it is known that the degrees of fat deposition in ultrasonography are significantly correlated with that in biopsy. Above all, there are many practical restrictions in executing invasive liver biopsy in patients with no symptoms; therefore, NAFLD was diagnosed by ultrasonography instead of liver biopsy in this study.18
The absolute criteria for the permissible alcohol intake in diagnosis of NAFLD has not been established. The National Institutes of Health (NIH) permits consumption of 140 g of alcohol per week for men and 70 g of alcohol per week for women. Becker et al21 reported that the risk of damage to the liver would increase if alcohol intake was larger than 168 g per week for men and 84 g per week for women; thus, the permitted limits in that study are different from those in this study. However, in studies conducted in Asian countries, the permissible alcohol intake for NAFLD was presented as 20-30 g per day for both men and women.22-24 In this study as well, the permissible range daily alcohol intake was limited to 20 g for both men and women, and there was no statistically significant difference in alcohol intake between the NAFLD group and the control group.
The index system was not induced based on the association between NAFLD and factors known from other studies but rather was based on only the statistical results of this study. Subjects included only those individuals who visited one health examination center of a general hospital; thus, the possibility exists that many persons with health problems were included in this population.
In addition, in case a discrimination tool is developed by using logistic regression analyses as the index system in this study, the problem of overfitting can occur. Therefore, the developed tool should be applied to other groups to calculate sensitivity, specificity, and the AUC of ROC curves. However, the subjects could not be divided in this study since the number of study groups was small.
Fatty liver of other causes should be excluded to diagnose NAFLD. However, due to the retrospective nature of this study that analyzed only the data on inquiries and tests of patients, all causes of NAFLD could not be excluded. We attempted to exclude as many as possible causes by checking all histories and excluding all patients with viral hepatitis and otherwise.
Unlike the ALT/AST ratios in this study, AST/ALT ratios is usually greater than 2 in cases of alcoholic fatty liver disease, thus, they are known to be useful indices for distinguishing alcoholic fatty liver disease from other diseases.25 However, for NAFLD, ALT values are generally higher than AST values, and if ALT/AST ratios >1.5 are converted into reciprocal numbers, they will be changed into AST/ALT ratios <0.667, which involves inconvenience. Therefore, in this study, AST/ALT ratios were changed into ALT/AST ratios. However, since ALT/AST ratios can be ≤1.5 in patients who have NAFLD with severe progressed fibrosis, severe cases may have lower points than mild cases in the index system.26
If the cut-off value in the index system is determined to be 3 points, its sensitivity and specificity is higher than 70%; thus, this system is considered to be a good tool to predict NAFLD through simple blood tests, physical examinations, and outpatient inquiries. If the cut-off value is determined to be 4 points, although sensitivity is reduced to 46.9%, specificity is increased to 92.3%; thus, it is thought that this index system can also be used as a tool to strongly recommend abdominal ultrasonography to patients with scores ≥4 points.
In conclusion, NAFLD can be sufficiently predicted by the simple and noninvasive index system induced in this study. By executing abdominal ultrasonography early in patients predicted with NAFLD, NAFLD will be early diagnosed, appropriately treated, and effectively managed.
Abbreviations
AFP
α-fetoprotein
ALP
alkaline phosphatase
ALT
alanine aminotransferase
AST
aspartate aminotransferase
AUC
area under the curve
BMI
body mass index
CI
confidence interval
DBP
diastolic blood pressure
HbA1C
hemoglobin A1C
HBsAg
hepatitis B surface antigen
HCV
hepatitis C virus
HDL-C
high-density lipoprotein cholesterol
NAFLD
nonalcoholic fatty liver disease
OR
odds ratio
ROC
receiver operating characteristics
SBP
systolic blood pressure
γ-GTP
γ-glutamyl transpeptidase