Effect of alcohol on the development of hepatocellular carcinoma in patients with hepatitis B virus-related cirrhosis: a cross-sectional case-control study
Article information
Abstract
Background/Aims
Whether alcohol intake increases the risk of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B virus (HBV) infection remains controversial. The aim of this study was to determine the effect of alcohol intake on the development of HCC.
Methods
Between January 2006 and August 2008, 146 patients with an initial diagnosis of HCC who were hospitalized in 3 major hospitals in the Incheon area were enrolled as cases. Another 146 cirrhotic patients, who matched the cases by age and sex, were enrolled as controls. All cases and controls were HBsAg positive, and had a history of lifetime alcohol intake.
Results
The cases and controls were aged 53±8 and 53±9 years (mean±SD), respectively, with each group comprising 118 males and 28 females. The basal laboratory data, distribution of Child-Pugh class, HBeAg positivity (31.5% vs. 37.7%), HBV DNA level (5.74±2.35 vs. 5.98±2.29 log10 copies/mL), and proportion with a lifetime alcohol intake of more than 292 kg (30.8% vs. 34.9%) did not differ between cases and controls. The cumulative alcohol intake and the proportion of heavy drinkers did not differ between the two groups in male patients.
Conclusions
Alcohol intake might not increase the risk of HCC in patients with HBV infection.
INTRODUCTION
Primary liver cancer is the fifth most common cancer and the third most common cause of cancer mortality in the world.1 The proportion of hepatocellular carcinoma (HCC) is 85~90% in primary liver cancers.2 Most of HCC cases occur in sub-Saharan Africa and Eastern Asia. The most common cause of HCC in these regions is hepatitis B virus (HBV).2
South Korea is located in Eastern Asia and has a high burden of HCC cases (male, 48.8/100,000; female, 11.6/100,000).2 Mortality of HCC is 22.7/100,000 (male, 34.1/100,000; female, 11.2/100,000) and HCC is the most common cause of death in 50~59 year old men.3 Although prevalence of HBV infection is decreasing after the universal HBV vaccination, HBV is the most common cause of HCC in South Korea.4
The risk factors for HCC in patients with chronic HBV infection are older age, male gender, presence of cirrhosis, family history of HCC, co-infection with hepatitis C virus (HCV) or hepatitis D virus, presence of hepatitis B e antigen (HBeAg), high level of HBV DNA, and high dose of alcohol intake.2 However, most of these are also risk factors for the development of cirrhosis in patients with chronic HBV infection. Cirrhosis is one of the most important risk factors for HCC and is considered a premalignant condition.5 In HBV-related HCC, 70~90% of cases have underlying cirrhosis.2 Therefore, for investigating risk factors for only HCC, it seems to be reasonable to enroll patients with cirrhosis rather than patients with chronic hepatitis without cirrhosis.
Alcohol plays an important role in the development of both cirrhosis6 and HCC,7,8 with a dose-effect relationship.9 However, although heavy alcohol intake is associated with the development of cirrhosis, there is still a controversy about a direct effect of alcohol on the development of HCC.2 Synergism between alcohol intake and HCV infection for the development of HCC was demonstrated in several reports. Patients with chronic HCV infection and heavy alcohol intake have a 2- to 4-fold risk for HCC compared to non-alcoholic patients with HCV.7,8,10 These findings revealed that there is a more than an additive interaction between alcohol and HCV infection. However, it is not clear whether alcohol intake adds to the risk of HCC in patients with chronic HBV infection.
This cross-sectional case-control study aimed to investigate whether heavy alcohol intake increase the incidence of HCC in patients with HBV-related cirrhosis.
PATIENTS AND METHODS
1. Patients
A total of 146 patients with an initial diagnosis of HCC, hospitalized in three major hospitals in the Incheon area, between January 2006 and August 2008, were enrolled as cases (HCC group). One hundred forty six cirrhotic patients, who matched the cases by age (±3 years) and sex, were enrolled as controls (cirrhosis group). Ethics Committees of each of the three hospitals approved this study, and written informed consents were obtained from all patients.
2. Diagnostic criteria of cirrhosis and HCC
Criteria for enrollment of all cases and controls were presence of hepatitis B surface antigen (HBsAg, RIA, Abbott Laboratory, Chicago, IL, USA) and absence of anti-hepatitis C virus (anti-HCV, RIA, Abbott Laboratory, Chicago, IL, USA). Diagnosis of cirrhosis was based on at least 2 of the following: 1) gastroesophageal varices on endoscopy, 2) cirrhotic surface or regenerating nodules of liver, and 3) splenomegaly by radiologic images (ultrasonography or computed tomography). Diagnosis of HCC was based on pathology or one of the following; 1) alpha-fetoprotein (AFP) ≥400 ng/mL and typical finding of HCC in radiologic image (abdominal computed tomography or abdominal magnetic resonance image or angiography), 2) AFP <400 ng/mL and typical finding of HCC in two kinds of radiologic images.3 In the HCC group, 45 cases were confirmed by pathology. All cases had underlying cirrhosis that was compatible to the above diagnostic criteria for cirrhosis.
3. Estimation of cumulative alcohol intake and laboratory data
All cases and controls were interviewed at each hospital about their lifetime alcohol intake. Lifetime alcohol intake was estimated using mean alcohol intake per day, days of alcohol intake per week, and years of alcohol intake. The amount of lifetime alcohol intake was categorized according to estimated alcohol dose: <146, 146~291, 292~583, 584~875, and ≥876 kg. HBeAg (RIA, Shinjin Medics Inc., Goyang, Korea), HBV DNA level (real time PCR, Abbott, Wiesbaden, Germany), and other laboratory data were checked at initial diagnosis in the HCC group and at enrollment in the cirrhosis group. However, if antiviral agents were used, the HBeAg, HBV DNA level, and laboratory data prior to antiviral therapy were taken. A total of 28 patients had oral antiviral treatment for median 17 (range:4-72) months.
4. Statistical analysis
We assumed the difference between the proportion of exposure (alcohol intake) in cases and that in controls were 0.14 (p1=0.26 in cases, p2=0.12 in controls) according to the result from Chen et al.11 The minimum sample size was calculated as 122 in each group, when alpha value was 0.05 and beta value was 0.2.
Continuous variables were expressed as the mean and standard deviation. The Student-t test was used for comparing continuous variables between the two groups. The Chi-square test was used for comparing categorical variables between the two groups. A P-value less than 0.05 was considered statistically significant. The SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
RESULTS
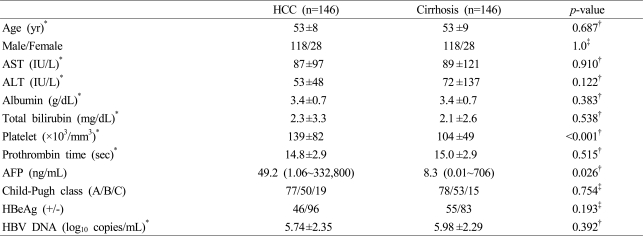
1. Clinical characteristics of the HCC and the cirrhosis groups
The mean age was 53±8 years among cases and 53±9 years among controls. One hundred eighteen males and twenty eight females were enrolled for each pair of cases and controls. In the HCC group, number of patients belonging to HCC stage according to TNM staging system were the following: 34 in stage I, 54 in stage II, 38 in stage III, and 20 in stage IV. There were no differences in the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, total bilirubin, prothrombin time, and the distribution of Child-Pugh class between the two groups. Platelet count was higher in the HCC group than in the cirrhosis group (139±82 vs. 104±49×103/mm3, p<0.001). The level of AFP was higher in the HCC group than in the cirrhosis group [49.2 (1.06~332,800) vs. 8.3 (0.01~706) ng/mL, p=0.026] (Table 1).
2. HBeAg and HBV DNA levels of the HCC and the cirrhosis groups
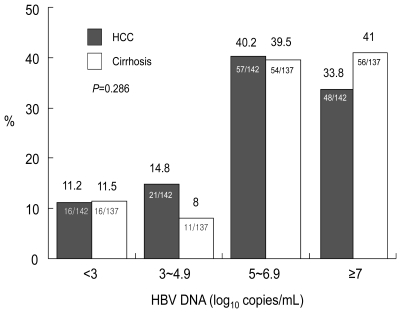
There were no differences in HBeAg positivity and mean HBV DNA level between the HCC and cirrhosis groups (Table 1). The levels of HBV DNA were categorized as <3, 3~4.9, 5~6.9, and ≥7 log10 copies/mL. There was no difference in the distribution of HBV DNA levels between the two groups (Fig. 1).
3. Amount of cumulative alcohol intake of the HCC and the cirrhosis groups
There was no difference in the distribution of cumulative alcohol intake between the HCC and cirrhosis groups (Fig. 2). The proportion of heavy alcohol drinkers defined as alcohol intake over than 80 g/day and more than 10 years, was 30.8% (45/146) in the HCC group and 34.9% (51/146) in the cirrhosis group. There was no difference in the number of heavy alcohol drinkers between the two groups (p=0.455).

Comparison of cumulative alcohol intake between the HCC and cirrhosis groups. The distribution of cumulative alcohol intake did not differ between the two groups. The proportion of heavy drinkers (lifetime alcohol intake greater than 292 kg) was 30.8% (45/146) in the HCC group and 34.9% (51/146) in the cirrhosis group (p=0.455).
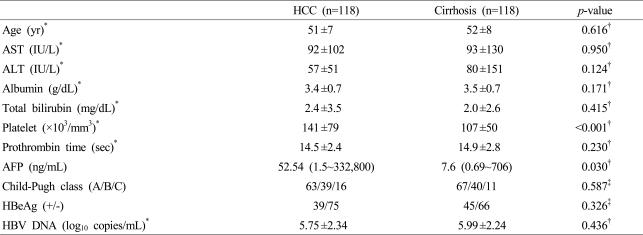
4. Clinical characteristics of HCC and cirrhosis groups in male patients
One hundred eighteen male patients were enrolled for each pair of cases and controls. The mean age was 51±7 years among cases and 52±8 years among controls. There were no differences in the levels of AST, ALT, albumin, total bilirubin, prothrombin time, and the distribution of Child-Pugh class between the two groups. Platelet count was higher in the HCC group than in the cirrhosis group (141±79 vs. 107±50×103/mm3, p<0.001). The level of AFP was higher in the HCC group than in the cirrhosis group [52.54 (1.5~332,800) vs. 7.6 (0.69~706) ng/mL, p=0.030] (Table 2).
5. HBeAg and HBV DNA levels between HCC and cirrhosis groups in male patients
There were no differences in HBeAg positivity and mean HBV DNA level between HCC and cirrhosis groups (Table 2). The levels of HBV DNA were categorized as <3, 3~4.9, 5~6.9, and ≥7 log10 copies/mL. There was no difference in the distribution of HBV DNA levels between the two groups (Fig. 3).
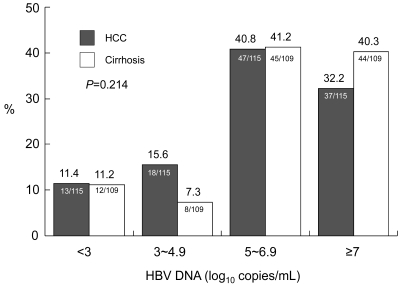
6. Amount of cumulative alcohol intake between HCC and cirrhosis groups in male patients
There was no difference in the distribution of cumulative alcohol intake between the HCC and cirrhosis groups (Fig. 4). The proportion of heavy alcohol drinkers was 38.1% (45/118) in HCC group and 40.7% (48/118) in cirrhosis group. There was no difference in the number of heavy alcohol drinkers between the two groups (p=0.689).

Comparison of cumulative alcohol intake by male patients between the HCC and cirrhosis groups. The cumulative alcohol intake did not differ between the two groups. The proportion of heavy drinkers (lifetime alcohol intake greater than 292 kg) was 38.1% (45/118) in the HCC group and 40.7% (48/118) in the cirrhosis group (p=0.689).
DISCUSSION
Chronic alcohol intake induces a variety of liver diseases such as fatty liver, liver fibrosis, cirrhosis, and HCC. In addition, it may increase the rate of progression to cirrhosis and HCC in patients with chronic HBV infection. A synergism between alcohol intake and HBV infection on the development of HCC was reported in patients with chronic HBV infection.7,12 However, other reports showed that alcohol intake did not add to the risk of HCC in patients with chronic HBV infection.13-15
To minimize error in the estimation of cumulative alcohol intake, in this study, alcohol dose was categorized according to the amount of a heavy alcohol drink. The dose of a heavy alcohol drink was defined as 292 kg of ethanol or more than this amount. The 292 kg of ethanol is compatible to the amount of a daily intake of 80 g ethanol for 10 years. This amount of ethanol usually induces alcoholic liver diseases in man, although a less amount of ethanol than this induces alcoholic liver diseases in woman.16 In this case-control study, the amount of past cumulative alcohol intake did not add to the risk of HCC in patients with HBV-related cirrhosis.
Because women are more susceptible to alcohol than men, woman can easily develop alcohol-mediated hepatotoxicity and severe forms of alcoholic liver disease.17 Therefore, a sub-group analysis including only men was done for determining alcohol effect on the development of HCC. The result of sub-group analysis also showed that alcohol intake did not add to the risk of HCC in male patients with HBV-related cirrhosis.
All findings in this study showed that alcohol intake did not add to the risk of HCC in patients with HBV-related cirrhosis. Even a very high alcohol dose did not add to the risk of HCC.
Many studies about alcohol effect on the development of HCC defined their own alcohol dose that possibly induces alcoholic liver disease. Therefore, it is very difficult to reach a conclusion about the effect of alcohol intake on the development of HCC. In addition, it is uncertain as to the amount of alcohol intake needed to induce advanced liver diseases or HCC. Some reported that an alcohol intake of more than 50-70 g/day is a risk factor for HCC.2 In patients with chronic HCV infection, alcohol intake of more than 60-80 g/day increased the risk of HCC from 2-4 times.7,8,10 However, it is unclear how much alcohol intake adds to the risk of HCC, and whether low or moderate alcohol intake also is a risk factor for HCC in patients with chronic HBV infection. In this study, the amount of alcohol intake was categorized to investigate the amount of alcohol needed to increase the risk of HCC. However, there was no difference in the distribution of cumulative alcohol intake between the HCC and cirrhosis groups.
The risk of HCC is higher in former drinkers, who have ceased alcohol drinking for up to 10 years, than in those who continue to drink alcohol.7,18 This paradoxical observation is explained by the fact that patients stop drinking due to the early signs of liver disease, but the underlying hepatic disease remains and influences the risk of HCC development. In contrast, patients who continue to intake alcohol in spite of signs of liver disease will often die from complications of alcoholic liver disease prior to the development of HCC.18 In the aspect of patients with chronic HBV infection, continuous alcohol drinking may aggravate pre-existing liver disease and finally shorten the life expectancy prior to the occurrence of HCC. Because of this hypothesis, even though alcohol can add to the risk of HCC, there is no definite evidence of carcinogenic effect of alcohol in many studies, and controversies remain as to whether alcohol can add to the risk of HCC in patients with chronic HBV infection.
The seropositivity of HBeAg is a risk factor not only for cirrhosis,19,20 but also for HCC.11,21 However, cirrhosis and HCC actually developed many years after HBeAg seroconversion,22 and an estimated 65% of liver complications occurred after HBeAg seroconversion.23 Generally, absence of HBeAg means inactive carrier status if ALT levels are normal and HBV DNA levels are low. However, some patients without HBeAg show an active liver disease state and have high levels of ALT and HBV DNA. Therefore, it is difficult to interpret the association between HBeAg status and clinical outcomes without considering ALT and HBV DNA levels. In this study, the seropositivity of HBeAg was not a risk factor for HCC in patients with cirrhosis. Because HBeAg is a common risk factor for both HCC and cirrhosis, it could not remain as a risk factor for HCC in patients with underlying cirrhosis.
A high level of HBV DNA is a risk factor not only for cirrhosis,24 but also for HCC.11,25 In addition, serum HBV DNA level at initial enrollment has a linear relationship with HCC risk; the higher the HBV DNA level, the more risk of HCC development.11 However, due to the fluctuating level of HBV DNA during a lifetime, the accuracy of one high HBV DNA level at a single time point may not predict the risk of HCC development. For example, it is not understandable that all young HBV carriers who have a very high level of HBV DNA at one single point develop HCC in later their life. In this study, HBV DNA level was not associated with the development of HCC in patients with cirrhosis. Because high HBV DNA level is a common risk factor for both HCC and cirrhosis, it could not remain as a risk factor for HCC in patients with underlying cirrhosis.
The role of HBV genotypes in the development of HCC is still controversial, because the prevalence of genotypes is variable according to geographic region. According to a U.S.A. study, although patients with HBV genotype C have an increased risk of HCC compared to patients with other genotypes, it was not statistically significant after accounting for other variables.26 However, studies from Taiwan showed a 3-to 6-fold increased risk of HCC in patients with HBV genotype C compared to patients with genotype B.27,28 Because the prevalence of genotype C in Korea is over 99% in patients with chronic HBV infection,29 this study did not evaluate the impact of HBV genotype on the development of HCC.
Cirrhosis has been presented as a strong risk factor for HCC in patients with chronic HBV infection. It was associated with a 3.6-fold increased risk of developing HCC, in a U.S.A. study26 and nearly a 10-fold increased risk of HCC, even after accounting for other markers of disease severity such as elevated ALT or HBV DNA levels, in studies from Taiwan and China.11,30 Cirrhosis also has been presented as a strong risk factor for HCC in patients with alcoholic liver disease.31 Because cirrhosis, independent of its cause (alcohol, HBV, HCV, etc), is associated with a high risk of HCC, some hepatologists think that the high rate of HCC in patients with chronic HBV infection may merely reflect the fact that HBV is a common cause of cirrhosis.32 In addition, alcohol does not directly induce HCC, but just induces cirrhosis, which predisposes to the development of HCC.5 Therefore, because all cases and controls had cirrhosis, this study could not find a risk factor for HCC.
In the present study, some limitations exist in evaluating the risk of HCC. First, in quantifying alcohol intake, most studies depend on interviews with patients or their families. However, this method may lead to an invalid estimate of alcohol intake due to many biases.33 Although interviews for quantity of alcohol intake were performed by trained individuals, there remains uncertainty in quantifying lifetime alcohol intake. Second, diagnosis of cirrhosis was clinically determined by endoscopy and imaging studies. Although liver biopsy is the gold standard for diagnosis of cirrhosis, it is dangerous to patients with decompensated cirrhosis and not an ethical tool for the aim of this study. Third, if history of the amount of alcohol intake after the development of cirrhosis was taken for each pair of cases and controls, effect of alcohol on the development of HCC was more accurately determined. However, it was impossible to know when each case and control developed cirrhosis. Fourth, this study was designed as case-control study. Although cohort study has better statistic power than case-control study, it needs long term follow up for the observation of HCC occurrence. In addition, alcoholics frequently drop out of study protocol. Therefore, further study that has more powerful statistics and can track patients more thoroughly is needed.
In conclusion, this case-control study showed there were no significant differences in cumulative alcohol intake, HBeAg positivity, and HBV DNA levels between the HCC and cirrhosis groups. Prospective cohort study is needed to clarify the risk factors for development of HCC in patients with HBV-related cirrhosis.
Acknowledgements
This study was supported by the Scientific Research Fund from the Korean Association for the Study of the Liver.
Abbreviations
AFP
alpha-fetoprotein
ALT
alanine aminotransferase
AST
aspartate aminotransferase
HBV
hepatitis B virus
HBeAg
hepatitis B e antigen
HBsAg
hepatitis B surface antigen
HCC
hepatocellular carcinoma
HCV
hepatitis C virus