The factors associated with longitudinal changes in liver stiffness in patients with chronic hepatitis B
Article information
Abstract
Background/Aims
Liver stiffness (LS) as assessed by transient elastography (TE) can change longitudinally in patients with chronic hepatitis B (CHB). The aim of this study was to identify the factors that improve LS.
Methods
Between April 2007 and December 2012, 151 patients with CHB who underwent two TE procedures with an interval of about 2 years were enrolled. Ninety-six of the 151 patients were treated with nucleos(t)ide analogues [the antiviral therapy (+) group], while the remaining 55 patients were not [the antiviral therapy (-) group]. The two groups of patients were stratified according to whether they exhibited an improvement or a deterioration in LS during the study period (defined as an LS change of ≤0 or >0 kPa, respectively, over a 1-year period), and their data were compared.
Results
No differences were observed between the antiviral therapy (+) and (-) groups with respect to either their clinical characteristics or their initial LS. The observed LS improvement was significantly greater in the antiviral therapy (+) group than in the antiviral therapy (-) group (-3.0 vs. 0.98 kPa, P=0.011). In the antiviral therapy (+) group, the initial LS was higher in the LS improvement group (n=63) than in the LS deterioration group (n=33; 7.9 vs. 4.8 kPa, P<0.001). However, there were no differences in any other clinical characteristic. In the antiviral therapy (-) group, the initial LS was also higher in the LS improvement group (n=29) than in the LS deterioration group (n=26; 8.3 vs. 6.5 kPa, P=0.021), with no differences in any other clinical characteristic.
Conclusions
A higher initial LS was the only factor associated with LS improvement in patients with CHB in this study.
INTRODUCTION
Chronic hepatitis B (CHB) is a chronic liver inflammation that creates fibrous tissue and leads to architectural distortion of the liver. If liver fibrosis progress to cirrhosis, complications arising from portal hypertension and functional hepatocyte loss develop. Furthermore, decompensated liver cirrhosis and hepatocellular carcinoma, the end points of liver fibrosis, directly threaten life. However, liver fibrosis is thought to be reversible if optimal management is started timely. The long-term use of oral antiviral agents can reduce liver fibrosis by suppressing hepatitis B virus (HBV) DNA levels and normalizing alanine aminotransferase (ALT) levels in CHB patients in the immune reactive phase.1,2 In addition, liver fibrosis is also reduced in CHB patients exhibiting spontaneous regression of the immune reactive state or a persistent inactive HBV carrier state.3,4
The prognosis of CHB patients is dependent on the extent and rate of progression of fibrosis. Therefore, knowledge of the precise stage of fibrosis is important in the contexts of treatment and determining treatment efficacy. Liver biopsy is considered as the gold standard for estimating degree of fibrosis, but is less than satisfactory for repeat evaluations due to its invasiveness, patients' discomfort, and risk of serious complications (0.3-0.5%), including death (0.03-0.1%).5,6 In addition, a small portion of liver tissue cannot represent the whole liver and the interpretation of results is subject to significant intra- and inter-observer variability.2
Accordingly, there is a need for a safe, repeatable non-invasive means of measuring the stage of liver fibrosis. Scoring systems based on serum markers of fibrosis and degree of fibrosis as determined by combinations of several different blood tests have been investigated.7,8 However, reported accuracies for the differentiation of moderate and severe fibrosis were not acceptable. In addition, several technical and patient related factors tend to cause under- or overestimations of fibrosis stage.
Several years ago, the FibroScan was introduced for the evaluation of liver fibrosis. It uses an ultrasound-based technique, known as transient elastography (TE), to measure the speeds of propagation of shear waves through the liver, which are directly associated with liver stiffness (LS).9,10,11 The TE technique has several merits as it is rapid, objective, safe, and repeatable. Furthermore, TE measures approximately 1/500 of the liver's total mass, and thus, reduces sampling errors. In addition, the intra- and inter-observation coefficients of variation are 3.2% and 3.3%, respectively, indicating very good reproducibility. Furthermore, recent reports showed that the measurement of LS by TE accurately predicts the presence of histological fibrosis in patients with liver disease of various etiologies, such as, CHB,1 chronic hepatitis C (CHC),12,13,14 primary biliary cirrhosis,15 and primary sclerosing cholangitis.16
Recently, a small number of longitudinal studies on TE examined its repeatability and relation with fibrosis improvement in CHB.1,17 Among them, some patients had no improvement of LS in spite of antiviral therapy.17 There are few studies about factors that improve LS in patients with CHB. In the present study, longitudinal LS change, presumed to represent hepatic fibrosis, were assessed in CHB patients treated with or without oral antiviral agents and factors associated with improved LS were investigated.
PATIENTS AND METHODS
Patients
Between April 2007 and December 2012, 224 patients with CHB underwent TE twice with an interval of about 2 years. A schematic of patient enrollment is provided in Figure 1A. All had hepatitis B surface antigen for more than 6 months. Seventy-three patients were excluded for: 1) concomitant hepatitis C virus (HCV) infection; 2) the initiation or cessation of oral antiviral therapy between the two TE exams; 3) excessive alcohol abuse of over 40 g/day; or 4) an ALT level of over 80 IU/mL at the time of first TE. Finally, 151 patients with CHB were included. Ninety-six received oral antiviral therapy, but the other 55 patients were not treated by oral antiviral therapy due to a low HBV DNA level (HBV DNA < 10,000 copies/mL in cirrhosis and < 100,000 copies/mL in chronic hepatitis) or a low ALT level (ALT ≤ 40 IU/mL in cirrhosis and ≤ 80 IU/mL in chronic hepatitis) as directed by the Korean National Health Insurance.

(A) Schematic diagram of patient enrollment. (B) The time point of LS measurements. In the antiviral therapy (+) group, the initial LS measurement was made after serum ALT had decreased to lower than 80 IU/mL. In the antiviral therapy (-) group, the initial LS was measured whenever serum ALT was lower than 80 IU/mL. Follow-up LS was measured at about 2 years after the initial LS. Annual LS changes were calculated by subtracting the follow-up LS value from the initial LS value, dividing the result by the number of months elapsed between the initial and follow-up LS measurements, and then multiplying by 12.
Liver cirrhosis was diagnosed based on clinical, laboratory, and radiologic findings according to clinical practice guidelines.18 This study was approved by the Gil Hospital Institutional Review Board (GAIRB2013-211).
LS measurement
LS was measured by TE (FibroScan®, Echosens, Paris) with the patient lying supine with the right arm fully abducted. Measurements were performed over the right lobe of the liver through the intercostal space. At least 10 valid TE readings were taken per patient, and median LS values were used for analysis. Results are expressed in kilopascals (kPa). Performance was considered optimal when the percentage of successful measurements with respect to the total number of acquisitions was at least 60% and the interquartile range to liver stiffness ratio was less than 0.03.
For the 96 patients that underwent antiviral therapy [the antiviral therapy (+) group], LS was initially measured when ALT decreased to lower than 80 IU/mL. In the 55 patients without antiviral therapy [the antiviral therapy (-) group], LS was initially measured when ALT was lower than 80 IU/mL. LS was re-measured at about 2 years after initial LS in all 151 patients (Fig. 1B). LS change per year was used to determine whether LS had improved or deteriorated. LS change per year was calculated by subtraction the second LS value from the first LS value dividing by the number of intervening months and multiplying by 12. When the LS change per year was ≤ 0, LS was considered improved, and if was > 0 was consider to have deteriorated (Fig. 1B).
Biochemical, hematologic, and virologic examinations
Routine laboratory tests, including liver function testing, were performed at first TE and then serially at 3-4 month intervals. Hepatitis B envelop antigen (HBeAg) (ADVIA centaur®sp, Siemens Medical Solutions, NY, USA) and HBV DNA levels (m2000 system, Abbott Molecular Inc., Des Plaines, IL, USA) were checked at first TE and then serially at 6 month intervals.
Statistical analysis
Statistical analysis was performed using a commercial software package (SPSS, version 13.0, SPSS Inc., Chicago, IL, USA). Values are presented as means±standard deviations or as medians and ranges. The analysis was conducted using the Student's t-test or the Mann Whitney U-test. Qualitative values are presented as numbers (%) and analyzed using the chi-square test or Fisher's exact test. LS changes were compared using the paired sample t-test. The multivariate analysis was performed using binary logistic regression analysis on variables found to be significant by univariate analysis (P<0.1). P-value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the patients
The pretreatment mean levels of laboratory data in 96 patients who received antiviral therapy [the antiviral therapy (+) group] were AST of 173±249 IU/L, ALT of 268±403 IU/L, total bilirubin of 2.1±3.3 mg/dL, albumin of 3.9±0.6 g/dL, platelet count of 173±77 ×103/mm3, and HBV DNA of 7.0±1.2 log10 copies/mL. The first TEs were checked after mean periods of 34±28 months after antiviral therapy in the antiviral therapy (+) group.
At the first TE, the antiviral therapy (+) group (n=96) had a higher rate of HBeAg (+) than the antiviral therapy (-) group (n=55) (39.5% vs. 16.9%, P=0.003). On the other hand, mean HBV DNA level was higher in the antiviral therapy (-) group than in the antiviral therapy (+) group (4.0±2.6 vs. 2.7±2.8 log10 copies/mL, P=0.008), because HBV DNA level had already been reduced by antiviral therapy in the antiviral therapy (+) group at first TE. These two groups did not differ in terms of age, gender, prevalence of liver cirrhosis, interval between the two LS exams, or laboratory findings (Table 1).
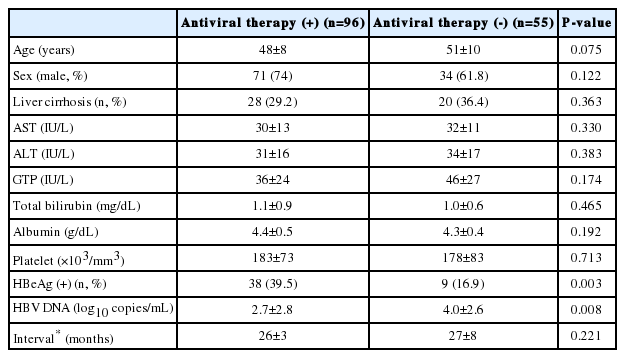
Comparison of the baseline characteristics of the antiviral therapy (+) and (-) patient groups at first transient elastography
In the antiviral therapy (+) group, patients were treated by lamivudine (LAM) (n=49), adefovir (n=12), clevudine (n=4), or entecavir (ETV) (n=31). Among these, 24 patients developed antiviral resistance and then antiviral agents were changed during the study period.
Initial LS values and LS changes
Mean initial LS was 10.6 kPa in the antiviral therapy (+) group and 11.5 kPa in the antiviral therapy (-) group (Fig. 2), which was not a significant difference (P=0.614). However, mean improvement in LS per year was higher in the antiviral therapy (+) group (-3.0 vs. 0.98 kPa, P=0.011) (Fig. 3). In the antiviral therapy (+) group, LS improved in 63 patients (65.6%), whereas in the antiviral therapy (-) group, LS improved in 29 patients (52.7%). However, these improvement rates were not significantly different (P=0.119).
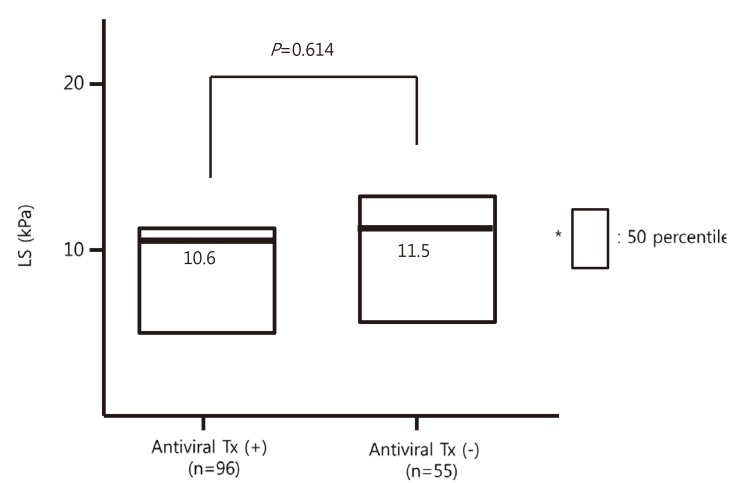
Comparison of initial liver stiffness values in the antiviral therapy (+) and (-) groups. The initial LS values were similar between the two groups.
Patients' characteristics and LS value according to whether LS is improved or deteriorated in antiviral therapy (+) group
In antiviral therapy (+) group, 63 patients achieved an LS improvement and in 33 patients LS deteriorated. No differences were found in the clinical characteristics and laboratory data between LS-improved and LS-deteriorated group (Table 2).
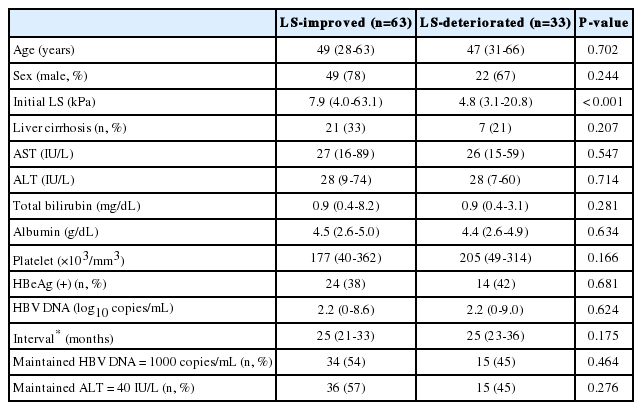
Characteristics of the patients according to whether they exhibited an improved or deteriorated liver stiffness in the antiviral therapy (+) group
Median LS value at first TE was higher in LS-improved than in LS-deteriorated group (7.9 vs. 4.8 kPa, P<0.001). The proportions of patients who maintained a HBV DNA level below 1000 copies/mL or a normal ALT level (≤40 IU/L) during the study period were similar in these two groups (Table 2). The median changes of LS in patients with or without maintained HBV DNA level below 1000 copies/mL were -1.95 and -0.4 kPa respectively (P=0.480).
Patients' characteristics and LS value according to whether LS is improved or deteriorated in antiviral therapy (-) group
In the antiviral therapy (-) group, 29 patients achieved an LS improvement and in 26 patients LS deteriorated. No differences were found in the clinical characteristics and laboratory data between LS-improved and LS-deteriorated group (Table 3).
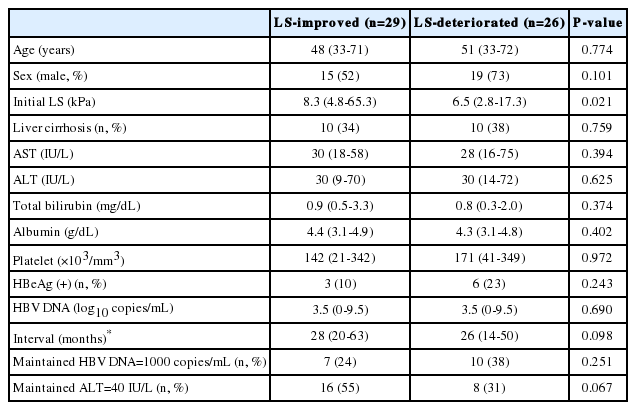
Characteristics of the patients according to whether they exhibited an improved or deteriorated liver stiffness in the antiviral therapy (-) group
Median LS value at first TE was higher in LS-improved than in LS-deteriorated group (8.3 vs. 6.5 kPa, P=0.021). The proportions of patients who maintained a HBV DNA level below 1000 copies/mL during the study period were similar in these two groups. However, the proportion of patients who maintained a normal ALT level (≤40 IU/L) during the study period was slightly higher in LS-improved group (55% vs. 31%, P=0.067) (Table 3).
Multivariate analysis and the factors that improved LS
Variables that had P-value less than 0.1 in univariate analysis and factors about treatment response such as maintained HBV DNA ≤ 1000 copies/mL and maintained ALT ≤ 40 IU/L, were used for multivariate analysis. Only a higher initial LS value was found to be significantly associated with LS improvement in the antiviral therapy (+) group [odd ratio (OR): 1.186, 95% confidence interval (CI): 1.048-1.342, P=0.007], and tended to be associated with LS improvement in antiviral therapy (-) group (OR: 1.114, 95% CI: 0.997-1.244, P=0.056).
DISCUSSION
Many authors have concluded that liver fibrosis can be reversed if the underlying cause of liver disease is removed. In CHC, the achievement of sustained virologic response (SVR) by interferon (INF) lowered the rate of fibrosis progression.19 In a long-term HCV study, fibrosis was improved in the majority patients, and even cirrhosis could be reversed in patients with SVR by INF-based therapy.20,21,22,23 Similar results were observed using LS measurements in CHC patients treated with INF-based therapy, that is, patients with SVR showed a significant reduction in LS versus those without SVR.24,25
HBV is rarely eradicated by antiviral treatment, but the sustained suppression of HBV replication can improve liver disease from the perspectives of blood chemistry and histology.26 Long-term treatment with LAM achieved improvements of histology in HBeAg+ve and HBeAg-ve patients, and even cirrhosis patients.27,28 However, long-term therapy with LAM can result in the development of resistance, and patients who developed resistance showed greater disease progression than those that did not develop resistance.27 Unlike LAM, ETV and tenofovir have low resistance rates. In ETV and tenofovir studies, the majority of patients treated with ETV or tenofovir showed significant improvements in liver histology and Ishak fibrosis scores.29,30,31 These studies demonstrate that regression of fibrosis is possible if HBV replication is successfully inhibited during long-term antiviral therapy. In a study on the use of LS measurements to evaluate changes in fibrosis, LS values significantly improved in CHB patients treated with ETV for 12 months.2 In the present study, although several types of oral antiviral agents were used, LS significantly improved after about 2 years of antiviral therapy. Although 24 cases of drug resistance occurred during the study period, the institution of rapid rescue therapy prevented LS deterioration.
In the present study, 55 patients did not receive antiviral therapy due to a low viral load or a low ALT level in accord with the requirements of the Korea National Health Insurance. Thirty-nine of these patients had a low HBV DNA and a low ALT level (data not shown), and 16 had a high HBV DNA but a low ALT level (data not shown). These patients was inhomogeneous in terms of natural history of chronic HBV infection.26 Some of our patients might have been in the immune tolerance phase, and others might have been inactive HBV carriers or in the active state. Furthermore, because no liver biopsy was performed in most of our patients, analyses by disease status were not possible. Generally speaking, these patients were expected to remain in a stable disease state with respect to fibrosis, because they had a stable HBV DNA or ALT level during the study period. However, our antiviral therapy (-) group had a mean LS deterioration of 0.98/year.
Only limited data is available on the natural course of CHB patients during the stable disease state. Contrary to the general perception that fibrosis does not progress during the stable disease state, we found that liver fibrosis showed a tendency to progress. However, as no pathologic diagnosis was performed in the present study, it was not known whether these patients were really in a stable state or not. Therefore, further study is needed to determine which patients show fibrosis progression by liver biopsy and which patients in the stable disease state benefit from antiviral therapy.
Although LS is a physical parameter that is mainly associated with fibrosis, it is also affected by other factors that influence liver elasticity, such as, inflammation, edema, and vascular congestion.32,33,34,35,36 In previous reports, LS was found to be affected by serum levels of total bilirubin, ALT, gamma-glutamyl transpeptidase, platelet count, albumin, international normalized ratio, and old age.1,10,11,37 Among these, serum ALT level was found to most importantly affect LS, more specifically, LS value and ALT levels were found to be positively related.32,38 Therefore, LS values should be cautiously interpreted when serum ALT is elevated. In the present study, patients with an ALT level >80 IU/mL were excluded to prevent ALT values affecting results.
By univariate analysis, a higher baseline LS value was significantly associated with an annual LS improvement in anti-viral therapy treated and untreated patients. By multivariate analysis, a higher baseline LS value was a significant factor only in the antiviral therapy (+) group, but a marginal association was observed in the antiviral therapy (-) group. Few reports are available on factors that improve LS in patients with chronic hepatitis. In one report, a higher LS value was found to be associated with a significant improvement in LS in patients with CHC treated with an INF-based therapy.25,39 In addition, a higher initial LS value was also found to be a significantly associated with an LS improvement in CHB patients treated with oral antiviral agents.17 A high initial LS value might be the result of elevated necroinflammatory activity, and more significant reductions in LS values were observed in the patients with an initial high LS value after the resolution of inflammatory activity.17,25,39 However, we excluded patients with an ALT level exceeding 80 IU/L, and therefore, the influence of ALT level on LS value was reduced in the present study. Therefore, LS value in this study is more convincing compared to previous studies. Further large scale studies using LS examinations and liver biopsy are required to prove this result.
Fung et al.17 performed a study with a 3-year follow-up on 316 untreated CHB patients, and reported only patients that maintained ALT within the normal range during the study period achieved an LS improvement. However, in the present study, the maintenance of a normal ALT level during the study period was not found to be associated with LS improvement. In the antiviral therapy (+) group, antiviral agents successfully suppressed ALT in most patients. Furthermore, even when antiviral resistance developed and ALT increased, the implementation of rapid rescue therapy suppressed further ALT increases. In the antiviral therapy (-) group, ALT did not increase high enough to influence LS values. Forty-one patients that did not meet insurance guidelines at first TE, later showed an ALT level increase and received antiviral therapy during the study period (Fig. 1A). However, these patients were excluded as mentioned previously. Therefore, the maintenance of a normal ALT level may not have been a significant factor of LS improvement in the antiviral therapy (-) group.
In the present study, a sustained low HBV DNA level was not associated with LS improvement. In the antiviral therapy (+) group, antiviral agents had suppressed serum HBV DNA level at first TE, and thus, further decreases in HBV DNA or a sustained low level of HBV DNA may not have effected LS improvements. Furthermore, as mentioned above, even when antiviral resistance developed, rapid rescue therapy suppressed further HBV DNA elevation and biochemical breakthrough. In the antiviral therapy (-) group, the majority of patients had a sustained low HBV DNA level during the study period. Thirty-nine of these 55 patients (71%) had a HBV DNA level of <10,000 copies/mL throughout the study period, and thus, a sustained low level of HBV DNA could not influence LS improvements.
Some limitations of the study warrant consideration. First, the effect of antiviral therapy on LS improvement would be best assessed by dividing the study population into antiviral treated and untreated groups, but this presents moral issues. Accordingly, we separately analyzed an antiviral therapy (+) group that satisfied the insurance guidelines and an antiviral therapy (-) group that did not, and despite separate analyses, a higher initial LS was found to be a common factor for LS improvement by univariate analysis. Second, paired liver biopsies are needed to estimate improvements in fibrosis. However, this procedure is not permitted in CHB patients with stable disease, and patients on antiviral therapy are unwilling to undergo paired liver biopsy.
In conclusion, the only factor found to be associated with LS improvement in CHB patients with antiviral therapy and in patients with stable disease state for about 2 years was a higher initial LS value. Despite serial checking ALT and HBV DNA levels at multiple time points, the maintenance of a normal ALT level and a sustained low HBV DNA level were not found to be significantly associated with LS improvement. However, this result can apply only to CHB patients with successful antiviral therapy or with stable disease state. A further, large scale, liver biopsy and TE based study is needed to identify factors that improve LS.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
ALT
alanine aminotransferase
CHB
chronic hepatitis B
CHC
chronic hepatitis C
CI
confidence interval
ETV
entecavir
HBV
hepatitis B virus
HBeAg
hepatitis B envelope antigen
HCV
hepatitis C virus
INF
interferon
kPa
kilopascals
LAM
lamivudine
LS
liver stiffness
OR
odds ratio
SVR
sustained virologic response
TE
transient elastography
