Similar respiratory function including chronic obstructive pulmonary disease between non-alcoholic fatty liver disease and metabolic dysfunction-associated steatotic liver disease
Article information
Dear Editor,
We have read with great interest the multi-stakeholder consensus statement that redefined the nomenclature for fatty liver disease [1]. The transition from the term “Non-alcoholic Fatty Liver Disease” (NAFLD) to “Metabolic Dysfunction-Associated Steatotic Liver Disease” (MASLD) represents a pivotal moment in the understanding and communication of this prevalent condition [2]. However, it is necessary to ascertain whether the evidence accumulated in association with NAFLD can be applied in the same manner as the transition to MASLD [3].
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally, yet there is no effective treatment. Similar to steatotic liver disease (SLD), it remains a condition with high unmet needs. Although smoking has been identified as a cause of COPD, investigation into the diverse clinical features of COPD has been limited [4]. NAFLD is one of the comorbidities of COPD and is associated with its severity [5,6]. In addition, metabolic dysfunctions, including hypertension and dyslipidemia, are associated with the prevalence of COPD [7]. However, there is uncertainty about the differences between NAFLD and MASLD regarding respiratory function and the prevalence of COPD.
We investigated the respiratory function based on spirometry results in patients diagnosed with NAFLD and MASLD by abdominal ultrasonography. The health check-up database used in this study was derived from staff medical check-ups as required by the Occupational Health and Safety Law in Japan and as individual voluntary participation, not based on hospital data. We enrolled 34,073 Asian participants over 40 years of age, who underwent health check-up examinations from January 2010 to March 2020. Ultrasound extaminations were performed by certified sonographers, and all images were adequately recorded. The following information was obtained using a self-reported questionnaire: age, sex, current smoking habits, alcohol consumption, comorbidities, and medication use. We excluded 23,987 participants with multiple check-ups on the same subject, 173 participants with the presence of hepatitis B surface antigen or presence of anti-hepatitis C virus antibody, and 2,384 participants with moderate or heavy alcohol consumption (>20 g/day for female, >30 g/day for male) and a lack of data on alcohol consumption. Finally, we examined 7,529 participants who underwent abdominal ultrasound and spirometry tests (Supplementary Fig. 1).
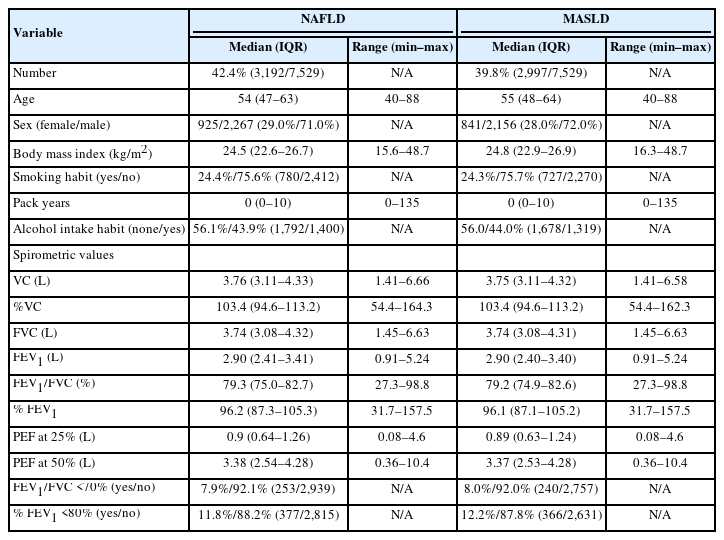
Out of 7,529 individuals, 50% (3,755 out of 7,529) were diagnosed with steatotic liver by ultrasonography. NAFLD was diagnosed in 42.4% (3,192/7,529), including 6.1% (195/3,192) of patients who did not fulfill the cardiometabolic criteria for MASLD. MASLD was diagnosed in 39.8% (2,997/7,529). In our cohort, the overlap rate between NAFLD and MASLD was 93.9%. This result was nearly identical to previous studies that reported approximately 95% of MASLD cases meeting the diagnostic criteria of NAFLD [3]. In our cohort, patients’ characteristics such as age, sex, body mass index, and habits of drinking and smoking were nearly identical for both NAFLD and MASLD cases.
We showed the spirometry results of patients with NAFLD and MASLD in Table 1. Vital capacity (VC) shows the maximum amount of air that can be expelled from the lungs. The median values for VC were almost identical in both NAFLD and MASLD groups, suggesting that neither condition significantly affects respiratory capacity. The values were comparable in both groups, indicating that the risk of restrictive pulmonary disease is not markedly elevated in either condition. Peak expiratory flow at 25% and 50% (PEF at 25% and PEF at 50%) are metrics assesing respiratory function during the middle of the exhalation process. Similar values in both NAFLD and MASLD cohorts suggest no significant specific impairment in respiratory function. Moreover, the presence of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <70% is a criterion for the diagnosis of COPD. This value was almost the same in both NAFLD and MASLD, 7.9% and 8.0%, respectively. In a large-scale cohort in Japan, the prevalence of COPD in individuals over 40 years of age has been reported to be 10.9% [8]. Additionally, the National Health and Nutrition Examination Survey in South Korea also reported a COPD prevalence of 10.0% among patients with NAFLD [9]. These results support the credibility of our health check-up data cohort.
In conclusion, the research evidence on respiratory dysfunction and COPD obtained in NAFLD could be applied to MASLD. The finding that one in ten patients with MASLD suffers from COPD, especially considering the widespread prevalence of MASLD, suggests that we should pay close attention to the potential for COPD in patients with MASLD.
Notes
Authors’ contribution
Conceptualization, T.T., and T.K.; methodology, T.T., and M.K.; software, D.N., and M.K.; validation, D.N., and M.K.; investigation, T.T., and T.K.; data curation, T.T.; writing—original draft preparation, T.T., T.K.; writing—review and editing, D.N. and M.K.; visualization, T.T.; supervision, H.T. and T.K.; project administration, T.T.; funding acquisition, T.T. and T.K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
T.K. received lecture fees from Janssen Pharmaceutical K.K., Taisho Pharmaceutical Co., Ltd., Kowa Company, Ltd., Otsuka Pharmaceutical Co., Ltd., Eisai Co., Ltd., ASKA Pharmaceutical Co., Ltd., AbbVie GK., EA Pharma Co., Ltd.
Acknowledgements
This research was partly supported by JSPS KAKENHI Grant Number JP23K15087, by Fukuoka Clinical Medical Research Award from the Medical Care Education Research Foundation, and by the Research Program on Hepatitis from the Japan Agency for Medical Research and Development, AMED, Grant Number 22fk0210094.
Abbreviations
NAFLD
non-alcoholic fatty liver disease
MASLD
metabolic dysfunction-associated steatotic liver disease
COPD
chronic obstructive pulmonary disease
SLD
steatotic liver disease
VC
vital capacity
PEF
peak expiratory flow
FVC
forced vital capacity
FEV1
forced expiratory volume in 1 second
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Study selection process.