Recent updates on pharmacologic therapy in non-alcoholic fatty liver disease
Article information
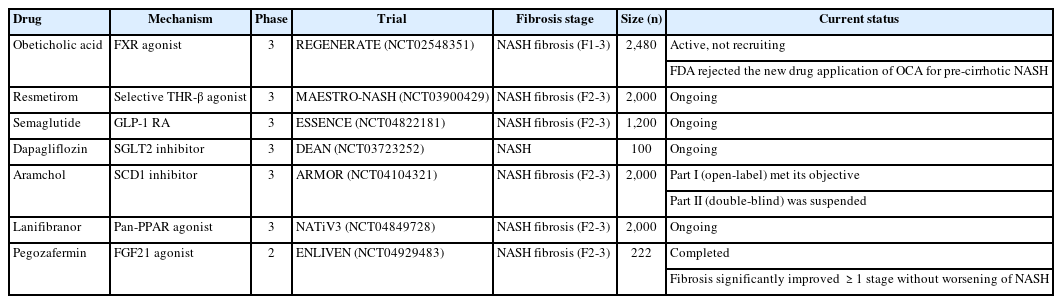
Since there are currently no US Food and Drug Administration (FDA)-approved drugs for the treatment of non-alcoholic steatohepatitis (NASH), various NASH treatments are under development for a wide range of targets. Several promising agents have recently failed in phase 3 trials, including selosertib [1], elafibranor, and cenicriviroc [2], and now five pharmacologic agents—obeticholic acid (OCA), resmetirom, aramchol, lanifibranor, and semaglutide—are being evaluated in large, histology-based phase 3 trials (Table 1).
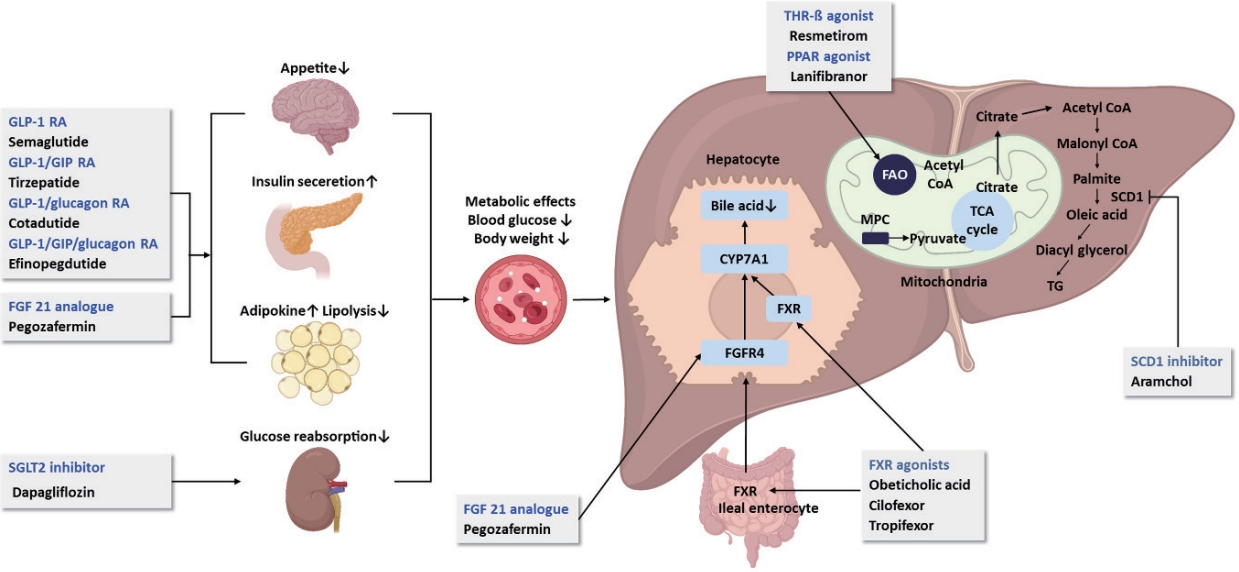
OCA, a farnesoid X receptor agonist, has been demonstrated to reduce hepatic fibrosis without worsening NASH in the 18-month interim analysis of the REGENERATE phase 3 trial [3], and its anti-fibrotic effect and long-term favorable safety profile have recently been validated [4]. However, in May 2023, the FDA rejected the new drug application of OCA for precirrhotic NASH, concerning safety issues such as hepatotoxicity, cholelithiasis, and pruritus, while its efficacy is modest. Resmetirom is a liver-targeted, thyroid hormone receptor β(THR-β)-selective agonist. Based on the promising results in the phase 2 clinical trial [5], phase 3 trials evaluating the efficacy of resmetirom in patients with NASH presenting with compensated cirrhosis (MAESTRO-NASH-Outcomes trial) and stage 2–3 fibrosis (MAESTRO-NASH) are ongoing. In the interim analysis of MAESTRO-NASH trial, which included 955 patients, histological NASH resolution and fibrosis reduction end points were achieved after 52 weeks of treatment [6]. Until now resmetirom is the only drug showing both NASH and fibrosis improvement in a phase 3 trial. The antidiabetic drugs, such as glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and glucose-dependent insulinotropic polypeptide (GIP), are attractive candidates for the treatment of NASH, considering their beneficial metabolic effects. These are incretins that stimulate insulin secretion from pancreatic β cells in response to food ingestion. Semaglutide, a GLP-1 RA, showed a significant dose-dependent NASH resolution without worsening of fibrosis in a phase 2 randomized controlled trial (RCT) involving patients with NASH and stage 1–3 fibrosis [7]. A phase 3 trial of semaglutide for NASH-related fibrosis (ESSENCE trial) is currently underway. However, in a recent phase 2 RCT for NASH-related cirrhosis, semaglutide did not improve fibrosis or NASH resolution, although it showed improvements in cardiometabolic parameters [8]. Effective drug therapy for NASH-related cirrhosis is challenging, as belafectin [9], selonsertib [1], and OCA also failed in this field. Tirzepatide, a dual agonist for GIP and GLP-1 receptors recently approved for type 2 diabetes mellitus, has been shown to reduce hepatic fat content as well as weight loss, suggesting a potential benefit for NASH [10,11]. Additionally, cotadutide, a dual receptor agonist of GLP-1/glucagon [12], and efocipegtrutide, a novel GLP-1/GIP/glucagon triple-receptor co-agonist [13,14], have shown potential as a treatment for NASH. Since glucagon receptors, unlike GIP and GLP-1, are highly expressed in the liver, glucagon agonism has significant impact on metabolism in the liver and extrahepatic tissues [15]. Another antidiabetic drug, dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, has been shown to reduce liver steatosis and fibrosis in small RCTs and meta-analysis [16,17]. A phase 3 trial (DEAN trial) to assess the efficacy and safety of dapagliflozin in improving NASH and metabolic risk factors is still ongoing. A phase 3 trial of aramchol (AMOR trial) was initiated on the basis of the observed safety and changes in liver histology in the phase 2b trial [18]. After the open-label part of the AMOR trial has met its objective, the manufacturer changed its proposed clinical studies with aramchol meglumine instead of aramchol free acid. Accordingly, initiation of the double-blind part is being suspended upon the manufacturer’s strategy. In a phase 2b clinical trial of lanifibranor in patients with NASH and significant fibrosis, lanifibranor achieved both endpoints of NASH resolution and fibrosis improvement, together with an improvement in lipid profile and insulin resistance [19]. A phase 3 trial of lanifibranor in patients with NASH and stage 2–3 fibrosis (NATiV3 trial) is currently ongoing. FGF21 is a non-mitogenic hormone that regulates glucose and lipid metabolism and modulates adiponectin secretion. Recently, pegbelfermin has failed to demonstrate significant reductions in liver fibrosis in patients with NASH [20,21]. In contrast, pegozafermin, a long-acting glycopegylated recombinant FGF21 analogue, led to significant improvements in fibrosis and NASH resolution in patients with stage 2–3 fibrosis (ENLIVEN trial) [22]. These promising results provide the basis for the advancement of pegozafermin into phase 3 development. An update on current pharmacological therapies for non-alcoholic fatty liver disease is summarized in Figure.
Several agents, including THR-ß-selective agonist, antidiabetic drugs, and FGF21 agonists show promise as new therapeutics for NASH, and their efficacy in improving both inflammation and fibrosis, with a long-term safety profile, is expected. Considering the heterogeneity of the disease and the various treatment responses observed in clinical trials for individual drugs, it is challenging to define effective drugs for all patients with NASH [23]. Accordingly, combination treatment or personalized treatment approaches may provide a higher response in the context of disease mechanisms.
Notes
Authors’ contribution
Young Chang drafted and revised the manuscript. Soung Won Jeong designed the concept and revised the manuscript. Jae Young Jang conducted the literature search and reviewed the manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund and the National Research Foundation of Korea (NRF) grant funded by the Korea government (2021R1I1A3059993, RS-2023-00251607).
Abbreviations
CYP
cytochrome P
FAO
fatty acid oxidation
FGF
fibroblast growth factor
FGFR
fibrosis growth factor receptor
FXR
farnesoid X receptor
GLP-1 RA
glucagonelike peptide-1 receptor agonists
GIP
glucose-dependent insulinotropic polypeptide
PPAR
peroxisome proliferator-activated receptor
MPC
mitochondrial pyruvate carrier
SCD
stearoyl coenzyme A desaturase
SGLT2
sodium glucose co-transporter 2
TCA cycle
tricarboxylic acid cycle
TG
triglyceride
THR
thyroid hormone receptor
FDA
US Food and Drug Administration
NASH
non-alcoholic steatohepatitis
OCA
obeticholic acid
RCT
randomized controlled trial