Taiwan Association for the Study of the Liver-Taiwan Society of Cardiology Taiwan position statement for the management of metabolic dysfunction-associated fatty liver disease and cardiovascular diseases
Article information
Abstract
Metabolic dysfunction-associated fatty liver disease (MAFLD) is an increasingly common liver disease worldwide. MAFLD is diagnosed based on the presence of steatosis on images, histological findings, or serum marker levels as well as the presence of at least one of the three metabolic features: overweight/obesity, type 2 diabetes mellitus, and metabolic risk factors. MAFLD is not only a liver disease but also a factor contributing to or related to cardiovascular diseases (CVD), which is the major etiology responsible for morbidity and mortality in patients with MAFLD. Hence, understanding the association between MAFLD and CVD, surveillance and risk stratification of MAFLD in patients with CVD, and assessment of the current status of MAFLD management are urgent requirements for both hepatologists and cardiologists. This Taiwan position statement reviews the literature and provides suggestions regarding the epidemiology, etiology, risk factors, risk stratification, nonpharmacological interventions, and potential drug treatments of MAFLD, focusing on its association with CVD.
INTRODUCTION
Metabolic dysfunction-associated fatty liver disease (MAFLD) and nonalcoholic fatty liver disease (NAFLD) are significant global health issues. In the general population, the incidence of MAFLD ranges from 15% to 30% [1]. The prevalence of NAFLD is approximately 55% in patients with type 2 diabetes mellitus (T2DM) and up to 80% in those with obesity [2,3]. The incidence rates of T2DM, hypertension, low high-density lipoprotein cholesterol levels, and hypertriglyceridemia are 9%, 8.4%, 9.6%, and 23.6%, respectively, in patients with biopsy-proven NAFLD [4].
The prognosis of hepatic outcomes in patients with MAFLD is associated with the severity of liver fibrosis [5]. Studies have demonstrated a significantly higher incidence of cirrhosis, hepatocellular carcinoma (HCC), and liver-related death in patients with NAFLD and fibrosis [6,7]. A study revealed an increase in cardiovascular events in patients with MAFLD [8]. The latest international consensus statements on the association between MAFLD and the risk of cardiovascular disease (CVD), which have been developed by experts from six continents, indicate that patients with MAFLD have higher cardiovascular events and mortality than individuals without MAFLD. In addition, CVD is the leading cause of death in patients with MAFLD [9].
Metabolic comorbidities are the leading risk factors for cardiovascular events and liver-related mortality in patients with MAFLD. T2DM intensifies the risks of CVD and chronic kidney disease due to increased insulin resistance (IR) [10]. The incidence of T2DM and hypertension also increases with the severity of MAFLD [11]. A meta-analysis revealed that T2DM, low high-density lipoprotein cholesterol levels, hypertriglyceridemia, and hypertension are significantly associated with a high risk of severe liver diseases, including cirrhosis, HCC, and liver-related mortality [12].
Position statement 1: MAFLD can lead to hepatic and extrahepatic morbidity and mortality.
Definition and diagnosis of MAFLD
In 2020, the international expert consensus recommended changing the term NAFLD to MAFLD. Compared with NAFLD, MAFLD adequately reflects similar pathophysiological mechanisms and cardiometabolic risk factors for fatty liver disease and CVDs, such as metabolic dysfunction, obesity, IR, and dyslipidemia [13]. MAFLD is diagnosed based on histological, imaging, or biomarker evidence of hepatic steatosis in patients with overweight/obesity, T2DM, or at least two metabolic risk factors (Fig. 1) [13].
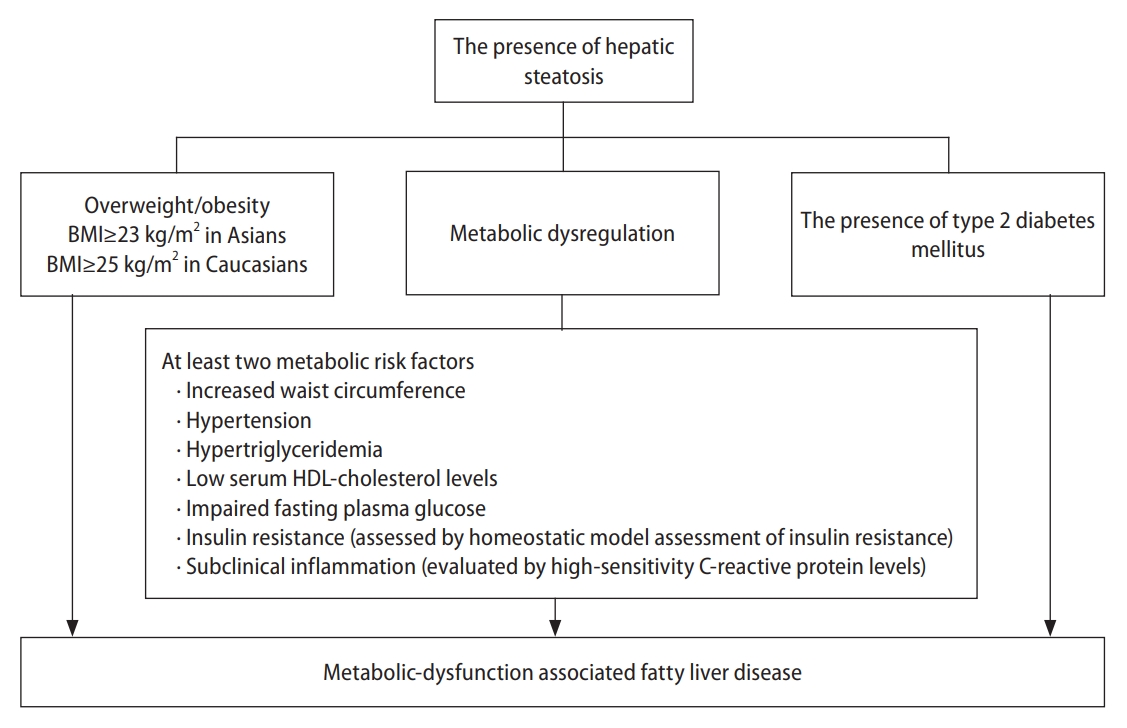
Definition of metabolic dysfunction-associated fatty liver disease. BMI, body mass index; HDL, high density lipoprotein.
Diagnostic tools
Liver biopsy remains the gold standard for the diagnosis and assessment of histological features in patients with NAFLD. However, the invasiveness of liver biopsy limits its routine use in clinical settings [14]. Ultrasound-based modalities are widely adopted as the first-line screening tools for hepatic steatosis; they have excellent performance for detecting moderate and severe steatosis, with a sensitivity and specificity of 84.8% (95% confidence interval [CI]: 79.5–88.9%) and 93.6% (95% CI: 87.2–97.0%), respectively [15]. Ultrasound-based transient elastography enables the quantitative evaluation of liver stiffness and steatosis. The area under the receiver operative characteristic curve of the ultrasonic controlled attenuation parameter for the detection of steatosis reached 0.95 in a previous study [16]. Magnetic resonance imaging-derived proton density fat fraction is the most sensitive noninvasive method for quantifying hepatic steatosis, with an area under the receiver operative characteristic curve of 0.95 [17]. Several noninvasive serum biomarkers, including the fatty liver index [18], hepatic steatosis index [19], NAFLD liver fat score [20], and lipid accumulation product [21], can be used to evaluate hepatic steatosis with moderate-to-good diagnostic performance (sensitivity: 86–93%, specificity: 40–71%) [22].
Position statement 2: MAFLD is defined as the presence of hepatic steatosis plus metabolic derangements.
Position statement 3: Abdominal ultrasonography is a useful and convenient tool for identifying hepatic steatosis.
MAFLD pathogenesis and risks
In 1998, the two-hit theory was proposed for the pathogenesis of NAFLD; it involves increased fat accumulation and the inflammatory cascade in the liver [23]. IR in the adipose tissue, muscle, and liver is a key factor in the first hit [24,25]. It is associated with energy imbalance caused by excessive caloric intake. Hepatic steatosis is caused by an imbalance between hepatic lipid storage and clearance, leading to excessive triglyceride-rich droplets in hepatocytes. In the second hit, the inflammatory cascade is overly activated by inflammatory cytokines, adipokines, lipotoxicity, endoplasmic reticulum stress, oxidative stress, and mitochondrial dysfunction [26-31]. Unresolved hepatic steatosis can progress to nonalcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and even HCC in severe cases [32,33]. Recent research has identified genetic factors, epigenetics, and gut microbiota dysbiosis as other MAFLD-associated molecular and metabolic elements [34-36], resulting in the “multiple-hit” pathomechanism [37].
Figure 2 presents the pathophysiological interaction between MAFLD and CVD. The “multiple hits” involved in the pathogenesis of MAFLD converge to a vicious cycle that promotes the development and progression of atherosclerosis and CVD [38,39]. In patients with MAFLD, the severity of hepatic steatosis and fibrosis is correlated with the coronary atheroma burden and atherosclerosis [40,41]. Moreover, inflammation and IR in MAFLD may increase the platelet count and the number of coagulation factors, which are associated with coronary arterial disease (CAD) [42] and venous thromboembolism (VTE).
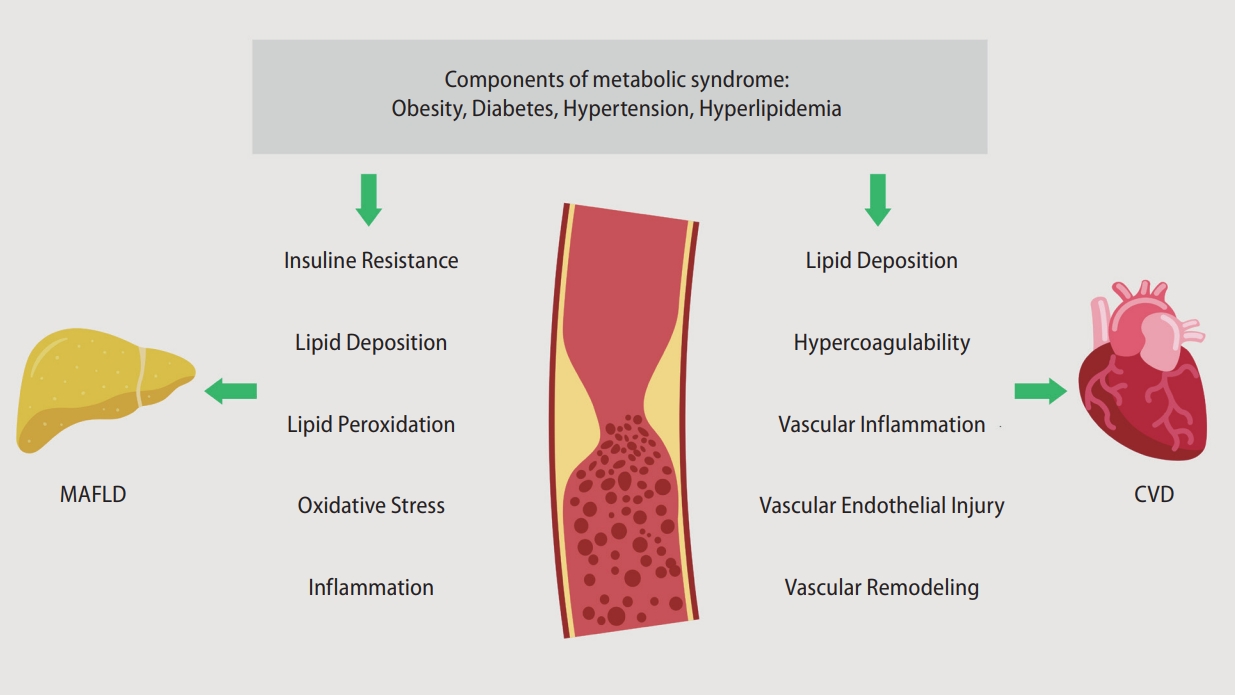
Pathophysiological mechanisms underlying the interaction between MAFLD and CVD. MAFLD, metabolic associated fatty liver disease; CVD, cardiovascular disease.
Metabolic disorders and genetic origins are involved in the development of MAFLD and CVD [43,44]. Multiple hits resulting from the interactions between genetic and environmental risk factors for MAFLD and CVD contribute to the occurrence of MAFLD and CVD (Fig. 3) [43,44].
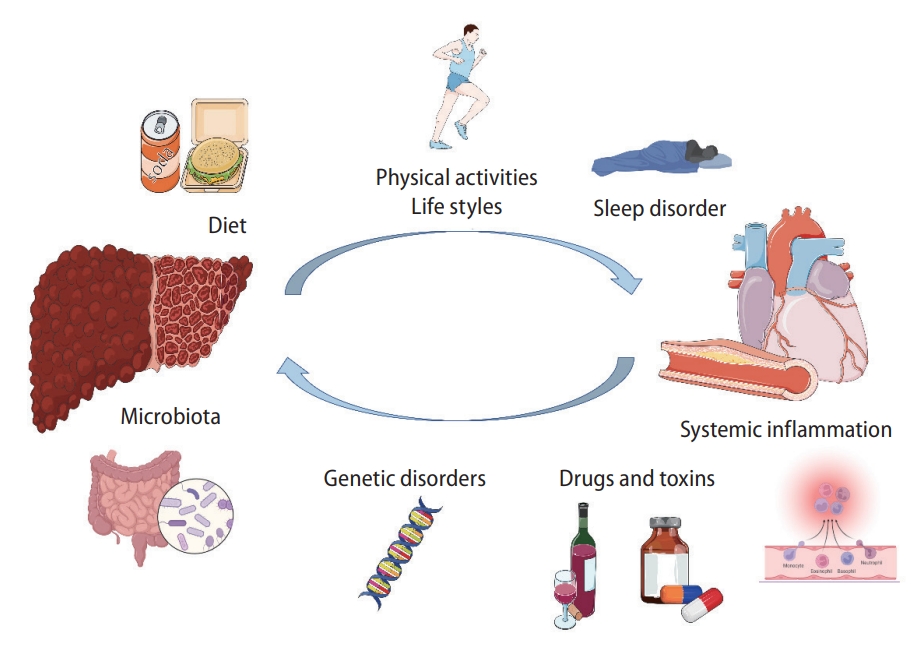
Illustration of the risk factors interplaying between the development of MAFLD and CVDs. MAFLD, metabolic associated fatty liver disease; CVD, cardiovascular disease.
Lifestyle factors
In genetically susceptible individuals, a sedentary lifestyle, a high sugar/saturated fat diet, metabolic derangements, and gut dysbiosis lead to MAFLD development and its progression [44]. Lifestyle changes, including limited intake of dietary fructose, are highly recommended [43].
Metabolic factors
Risk factors for MAFLD include male sex, advancing age, obesity, IR, T2DM, and hyperlipidemia, which are linked to gut dysbiosis [45]. IR is significantly involved in the pathogenesis of MAFLD and its progression to NASH, with T2DM being strongly associated with MAFLD, NASH, and CVD [46]. Cholecystectomy is an independent risk factor of MAFLD, which is attributable to altered bile acid enterohepatic circulation [47].
Genetic factors
Several genetic variants (PNPLA3, TM6SF2, and MBOAT7) can increase the susceptibility to NAFLD [48]. However, a Mendelian randomization analysis revealed no causal relationship between the NAFLD-associated PNPLA3 variant and CVD. Among the NAFLD-related genetic variants, TM6SF2 appears to be protective against VTE, whereas MBOAT7 may exert unfavorable effects [49].
Others
Other risk factors include steatogenic drugs, male sex, and infections. Coronavirus disease 2019; hepatitis C; acquired immunodeficiency syndrome; Helicobacter pylori-induced peptic ulcers; and periodontitis caused by Bacteroidetes, Candidatus Saccharibacteria, Firmicutes, and Proteobacteria worsen MAFLD [50].
Position statement 4: MAFLD and CVD have similar risk factors that exacerbate their progression. Identifying these risk factors is crucial for effective management and treatment.
Screening strategy for MAFLD in patients with CVD
Who should be screened?
Patients with MAFLD who have T2DM, central obesity, a sedentary lifestyle, and metabolic syndrome have a high risk of advanced fibrosis [51]. Moreover, the severity of fibrosis is associated with cardiovascular risk in patients with steatosis or steatohepatitis [52]. Thus, MAFLD surveillance should be considered in patients with CVD. For patients with subclinical atherosclerosis and multiple risk factors for CVD, MAFLD screening may be considered [53].
Position statement 5: MAFLD should be considered in patients with CVD, irrespective of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels.
Screening procedure
The screening tool should effectively identify patients with MAFLD who have advanced liver fibrosis. Transient elastography is more cost-effective than magnetic resonance elastography (MRE) for detecting advanced liver fibrosis, although its sensitivity and specificity are compromised [54]. Thus, in patients suspected of having advanced fibrosis or those with inconclusive sonography and transient elastography findings, MRE should be considered. Indirect serological biomarkers include AST levels, AST-to-platelet ratio, fibrosis-4 (FIB-4) score, NAFLD fibrosis score, and AST-to-ALT ratio. Direct serological biomarkers include the enhanced liver fibrosis test score and FibroMeter NAFLD test score [53].
Fibrosis assessment
Fibrosis assessment is crucial in patients with MAFLD. Primary care practitioners, gastroenterologists, cardiologists, and neurologists should screen for advanced fibrosis in patients with MAFLD and CVD. The FIB-4 index may be practical, as the calculation is straightforward and is based on widely available, simple, and cost-effective tests. As no single measurement or threshold value has high sensitivity and specificity (≥80%), a sequential algorithm having the FIB-4 index as the first-line test and liver stiffness measurement (LSM) as the second-line assessment is recommended. Figure 4 presents the algorithm recommended for MAFLD screening and liver fibrosis assessment among patients with CVD. The recommended algorithm is based on both clinical evidence and expert consensus. A meta-analysis revealed that a sequential combination of FIB-4 scores of <1.3 and ≥2.67 and subsequent LSM scores of <8.0 and ≥10.0 kPa could rule-in and rule-out advanced fibrosis, with a sensitivity of 66% (95% CI: 63–68%) and specificity of 86% (95% CI: 84–87%), respectively [55]. In another study, patients with FIB-4 scores of <1.3 had a low risk of HCC (0.05–0.21/1,000 person-years), whereas those with FIB-4 scores of >2.67 had a high risk of HCC (1.9–4.56/1,000 person-years) [56]. This sequential algorithm minimizes unnecessary tests and referrals, facilitates the timely identification of advanced fibrosis, and improves cost-effectiveness [53]. Therefore, several clinical guidelines recommended similar algorithms, such as American Gastroenterological Association (AGA)'s NASH Clinical Care Pathway [57], the American Association of Clinical Endocrinology (AACE)/American Association for the Study of Liver Diseases (AASLD) Clinical Practice Guideline for NAFLD [58], and The Japan Society of Hepatology (JSH)-The Japan Society of Gastroenterology (JSG) Clinical Practice Guidelines for NAFLD/NASH 2020 [59].

Algorithm for MAFLD screening and fibrosis assessment among CVD patients. MAFLD, metabolic associated fatty liver disease; CVD, cardiovascular disease; FIB-4, fibrosis-4.
Position statement 6: Determination of disease severity by noninvasive markers, preferably FIB-4, is recommended.
Identification and management of cardiovascular comorbidities in patients with MAFLD
The association between MAFLD and CVD risk is receiving increasing scientific and clinical research interest, and increasing evidence supports that patients with MAFLD have increased risks of CVD morbidity and mortality [60]. Advanced fibrosis and cirrhosis are associated with high liver-related death rates in patients with MAFLD [61], whereas mild fibrosis predisposes patients with MAFLD to risks of cardiovascular events and nonhepatic malignancies [62]. MAFLD serves as an indicator of high cardiovascular risk and contributes to CVD development. It can therefore be considered an important risk factor for CVD [63-65].
MAFLD and atherosclerotic cardiovascular diseases (ASCVD)
Approximately 10% of patients with MAFLD in primary care facilities have CAD [66]. Chinese and Taiwanese studies have suggested that MAFLD is associated with high risks of cardiovascular events and subclinical CAD, and that the ASCVD burden is substantial in patients with MAFLD [40,67,68]. The extent of steatosis increases the coronary atheroma burden in patients with MAFLD [69]. Moreover, liver fibrosis markers are associated with CAD progression [41]. MAFLD is also associated with worsened outcomes in patients undergoing coronary artery bypass grafting and percutaneous coronary angioplasty [70-72]. In patients with myocardial infarction (MI), concomitant MAFLD exacerbates the risk of cardiovascular events and death [73]. A large biobank analysis reported the association of MAFLD with cardiovascular and all-cause mortality [74]. Patients with both non–ST-segment elevation MI and MAFLD have a high risk of premature ventricular complexes and ventricular tachycardia [75].
MAFLD and arterial hypertension
High blood pressure may predict MAFLD onset independently of conventional risk factors [76]. A recent study in Taiwan revealed that patients with fatty liver have a high risk of prevalent and incident hypertension and/or diabetes. Moreover, the risk increases with an increase in the severity of fatty liver [11]. Another study suggested that effective hypertension control reduces the risk of MAFLD [77].
MAFLD and heart failure
In patients with heart failure (HF) with preserved ejection fraction (HFpEF), the prevalence of MAFLD is approximately 50% [78]. Patients with MAFLD have high left ventricular (LV) filling pressure in addition to a more fibrotic LV myocardium and worse global longitudinal strain [79]. Increased hepatic sinusoid resistance and venous return impairment can lead to a high normal cardiac output and high LV mass, which is characteristic of obstructive HFpEF. MAFLD may affect cardiac metabolism [80,81], and fibrosis may promote the formation of spontaneous portosystemic shunts, altering arterial blood flow and systemic vascular resistance in patients with HFpEF, which are associated with cirrhosis and advanced liver disease [82].
MAFLD and cardiac arrhythmias
The incidence of QT interval prolongation is high in patients with MAFLD and T2DM [83]. Ventricular arrhythmias, atrioventricular blocks, and atrial fibrillation (AF) are more frequent in patients with MAFLD [75,84]. After catheter ablation, liver fibrosis is linked to adverse atrial remodeling and recurrent AF in patients with MAFLD [85]. The Rotterdam study reported an association between AF and liver stiffness, but not steatosis [86,87]. The conflicting results may be attributed to heterogeneous patient backgrounds.
MAFLD and thromboembolic diseases
MAFLD is an independent risk factor for VTE [88], and 81% of patients with VTE have MAFLD [89]. The levels or activities of von Willebrand factor; factors VII–IX, XI, and XII [90]; and plasminogen activator inhibitor-1 are high in patients with MAFLD [91]. Patients with NASH have higher anticardiolipin immunoglobulin G levels than those with MAFLD [92], suggesting the association of thrombotic risks with liver fibrosis. Obesity is a VTE-associated risk factor in MAFLD [93,94]. However, the potential benefits of different interventions, such as bodyweight reduction, aerobic exercise, bariatric surgery, and anticoagulation medications, for VTE risk warrant further investigation [95,96].
Position statement 7: MAFLD increases the risks of hepatic-related and cardiovascular events, and it increases the risk of CVD.
Screening and management strategy of cardiovascular risks in patients with MAFLD
In patients with MAFLD, CVD risk screening and early management are recommended [97,98]. A regional, validated risk calculator can be used to stratify the 10-year ASCVD or CAD risk in these patients. In patients with a high risk of CAD or angina, stress or imaging tests for CAD should be considered [99]. If risk factors, such as hypertension, obesity, T2DM, and advanced age, are present, referral for echocardiography and natriuretic peptide testing should be considered in symptomatic cases [100]. Early referral to a cardiologist is highly recommended for symptomatic cases or patients with MAFLD who have high cardiovascular risk [99,100].
Position statement 8: In patients with MAFLD, cardiovascular risk screening and management are recommended. In symptomatic or high-risk cases, referral and multidisciplinary care involving cardiologists are highly recommended.
Linking care of MAFLD and CVD: Decreasing risks of CVD and liver cancer/HCC
Nonpharmacological management of MAFLD/NAFLD
Lifestyle modification
Lifestyle interventions that reduce bodyweight are crucial for managing NAFLD [101]. Approximately 5% weight loss is required to improve liver steatosis, and >10% weight loss is required for managing both liver steatosis and fibrosis [102,103]. However, sustained weight loss is challenging. Approximately 21.2% of patients with initial weight loss regained weight after a median follow-up of 32.3 months [104]. Thus, a multidisciplinary approach involving physicians, psychologists, behavioral therapists, dieticians/nutritionists, patients’ families, patient support groups, and digital support is pivotal for lifestyle interventions [105,106].
Dietary control
Excessive dietary intake of calories, saturated fats, refined carbohydrates, and sugar-sweetened beverages is common in patients with NAFLD and obesity [107-110]. Dietary macronutrients are involved in the pathogenesis of NAFLD [111]. For instance, fructose promotes hepatic steatosis and inflammatory signaling [107], and polyunsaturated fatty acids exhibit antiinflammatory effects [112]. The current guidelines of the European Association for the Study of the Liver (EASL) and the Asian Pacific Association for the Study of the Liver (APASL) recommend a hypocaloric diet (500–1,000 kcal deficit) [113,114]. Several trials support changing the amount and type of dietary carbohydrate/fat or adopting the Mediterranean diet, as both strategies can improve hepatic steatosis, regardless of weight loss [115,116]. Furthermore, the Mediterranean diet is effective in primary CVD prevention [115,117]. Regular coffee consumption is also associated with low risks of NAFLD and liver fibrosis [118,119].
Exercise
Exercise improves MALFD/NAFLD through various mechanisms, such as the upregulation of several signaling pathways, particularly those involving the peroxisome proliferator-activated receptor gamma (PPAR-γ) [120,121]. Exercise may downregulate mammalian target of rapamycin complex 1 signaling, further alleviating MAFLD/NAFLD [122]. Exercise training is beneficial for hepatic and cardiometabolic function in patients with MAFLD/NAFLD [123]. It improves vascular stiffness and endothelial dysfunction, thereby decreasing cardiovascular risk [124]. By reducing fibrosis, vigorous exercise improves the histological findings of NASH [125]. Regular and moderate exercise for at least 150 minutes per week or increasing activity levels for >60 minutes per week can ameliorate MAFLD/NAFLD [126].
Aerobic exercise, defined as continuous and rhythmic activities requiring the use of large muscle groups, is the primary training modality assessed in NAFLD exercise studies. By contrast, the benefit of resistance training remains controversial because of the heterogeneity of training intensity and protocols. A combination of aerobic and resistance training is expected to outperform either exercise modality [127,128]. Alternative activities, such as yoga, Pilates, and tai chi, have exhibited beneficial effects in pilot studies [129-131]. Updated guidelines of the AASLD and EASL strongly recommend any type of sustained individualized exercise for patients with MAFLD/NAFLD [126].
Bariatric surgery
Bariatric surgery leads to a sustained weight loss of up to 30% in patients with obesity, in addition to improving T2DM, NASH/NAFLD, morbidity, and mortality [132,133]. Patients undergoing bariatric surgery showed NASH resolution and fibrosis regression 5 years postoperatively [134]. Bariatric surgery also reduced CVD risk and CVD-associated morbidity in patients with obesity and NAFLD [135,136]. In addition, endoscopic bariatric and metabolic therapies (EBMT) improved aminotransferase levels and decreased NAFLD activity scores in patients with obesity and NAFLD [134,137,138]. However, well-designed prospective studies are warranted to assess the hepatic and cardiovascular benefits of EBMT in patients with NAFLD and obesity.
Position statement 9: Lifestyle modification constitutes the basic and important approach.
Position statement 10: Bodyweight reduction is the cornerstone of the nonpharmacological management of MAFLD; however, long-term bodyweight control remains a concern.
Pharmacological intervention for MAFLD
Although no drugs have been approved for MAFLD, the treatment of metabolic conditions closely associated with MAFLD may reverse IR, thereby ameliorating steatohepatitis and preventing fibrosis. Although lifestyle modification and weight loss are recommended as first-line interventions and can effectively reduce steatosis, inflammation, and fibrosis, they are often unsuccessful [102]. Therefore, pharmacological therapy may address the gap in treatments inhibiting MAFLD progression. Table 1 summarizes the investigated drugs for MAFLD. The use of approved antidiabetic drugs, including biguanides, glucagon-like peptide-1 receptor agonists (GLP-1RAs), dipeptidyl peptidase-4 inhibitors (DPP-4is), sodium-dependent glucose cotransporter-2 inhibitors (SGLT-2is), and PPAR agonists, has been investigated in patients with NASH [139,140]. Novel agents for NASH/NAFLD are in different phases of clinical development; their mechanisms of action include participation in de novo hepatic lipogenesis, mitochondrial fatty acid oxidation, inflammation, cell injury, collagen deposition, and fibrinolysis [141].
Vitamin E
Oxidative stress plays a key role in the pathogenesis of NASH; thus, vitamin E is justifiable as a therapeutic agent for NASH. Randomized controlled trials (RCTs) have been conducted in nondiabetic adults, children, and adolescents with biopsy-proven NASH [142-144]. Pooled analyses have demonstrated that vitamin E significantly decreases aminotransferase levels and improves the histological characteristics of NASH, except for liver fibrosis [144-146]. In an RCT involving patients with coexisting T2DM and NASH, 18 months of vitamin E supplementation histologically improved steatosis [147]. However, the role of vitamin E in NASH and advanced fibrosis or cirrhosis remains inconclusive.
The safety concerns of vitamin E should be considered. In one study, all-cause mortality was high in patients taking a high dose (>800 IU/day) of vitamin E [148]. Moreover, vitamin E increases the risk of HF in patients with vascular disease or T2DM [149] and the risk of prostate cancer in healthy men [150]. Although a high-vitamin E diet is associated with reduced stroke risk [151], it may significantly increase the risk of hemorrhagic stroke [152]. In summary, vitamin E supplementation at a daily dose of 800 IU may be considered in nondiabetic adults with biopsy-proven NASH. The associated risks and benefits should be fully discussed with each patient before initiating therapy.
Bile acids
The AASLD or EASL does not recommend ursodeoxycholic acid, a natural dihydroxy bile acid, for the treatment of NAFLD or NASH because of insufficient evidence regarding its beneficial effects on liver histology.
Obeticholic acid (OCA) is an analog of the bile acid chenodeoxycholic acid and a potent farnesoid X receptor agonist. Although the primary endpoints were met in the phase 2 FLINT trial and phase III REGENERATE trial of OCA, the U.S. Food & Drug Administration (US FDA) raised safety concerns regarding pruritus, high low density lipoprotein (LDL) levels, and limited changes in cardiovascular risk [153,154]. Consequently, the AASLD, EASL, and APASL do not recommend OCA for offlabel use for the treatment of NASH by [155-157].
Lipid-lowering agents
Statins may decrease LDL levels and cardiovascular risk in patients with NAFLD and NASH without liver decompensation. However, according to the AASLD and EASL, this treatment does not benefit or harm patients with liver disease [155-157]. Recent study has presented mixed findings regarding the role of PCSK9 inhibitors in managing earlystage NAFLD, emphasizing the need for extensive long-term research to ascertain their efficacy and safety [158].
Glucose-lowering agents
Metformin
Metformin is a biguanide with a mild insulin-sensitizing effect. It is traditionally the first-line therapy for T2DM. In patients with NAFLD unresponsive to lifestyle modifications, biochemical improvement was observed after metformin treatment [159]. Hepatic fat reduction with weight loss was also noted in a proportion of patients with NASH who were treated with metformin [160]. In an open-label trial, metformin in combination with rosiglitazone further improved liver histology in patients with NASH [161]. However, a meta-analysis of metformin trials did not reveal improvements in the liver disease activity score or fibrosis stage [162-164]. Overall, insufficient evidence supports the routine use of metformin in patients with NASH [164].
Pioglitazone
Pioglitazone was found to improve liver function, decrease hepatic fat, and improve NASH features in clinical trials and systemic reviews [165,166], regardless of the diabetic status [167]. Although weight gain was observed after pioglitazone therapy, data on other thiazolidinediones are limited [168]. In patients with T2DM and NASH, pioglitazone reduced CVD events [169].
GLP-1RAs
GLP-1RA is a new class of antidiabetic agents for T2DM that can improve weight loss, glycemic control, and liver enzyme levels by activating the gut-derived incretin pathway [170]. GLP-1RAs exhibit beneficial renovascular and cardiovascular effects on T2DM [171,172]. Histological findings of the phase 2 LEAN RCT revealed that patients with T2DM receiving liraglutide for 48 weeks had higher NASH resolution and lower fibrosis progression than those receiving placebo [173]. In a phase 2 trial of semaglutide, compared with placebo, 72-week semaglutide treatment resulted in significantly higher NASH resolution in patients with biopsy-proven NASH and F1–F3 liver fibrosis. However, the semaglutide trial did not reveal beneficial effects in improving the fibrosis stage [174]. In a systematic review and meta-analysis of patients with T2DM and NAFLD, GLP-1RAs effectively improved intrahepatic, visceral, and subcutaneous adipose tissue; liver function; body mass index; waist circumference; and glucose/lipid profiles but did not improve liver fibrosis markers, such as FIB-4 and NAS [175]. The main adverse events were mild-to-moderate gastrointestinal discomfort, such as poor appetite, constipation, diarrhea, and hypoglycemia, which resolved within a few weeks. Although a few small-scale studies have reported that GLP-1RAs are associated with NASH resolution and fibrosis regression, more large-scale studies are warranted.
SGLT-2is
SGLT-2is are antidiabetic agents that have extended benefits, and they are approved for reducing adverse outcomes in nondiabetic patients with HF and chronic kidney disease [176,177].
An observational study revealed that add-on treatment with 50 mg ipragliflozin for 45 weeks improved glycemic control and normalized ALT levels in patients with T2DM and NAFLD who were unresponsive to incretin-based therapy [178]. SGLT-2is also improved glycemic control and liver function in patients with T2DM and NAFLD and exclusively caused weight loss [179,180]. The efficacy of canagliflozin, dapagliflozin, and empagliflozin for NAFLD or NASH has been investigated in RCTs involving patients with T2DM with or without NAFLD, and the hepatic benefits, including aminotransferase, steatosis, and fibrosis improvements, of SGLT-2is have been noted [175,181-183]. Overall, SGLT-2is have exhibited positive effects on hepatic steatosis in meta-analyses; however, their effect on liver fibrosis requires further investigation [184-186].
DPP-4is
DPP-4 inhibition reduces glucagon levels, delays gastric emptying, stimulates insulin release, and augments pancreatic beta-cell regeneration [187]. DPP-4is may alleviate T2DM-related microvascular complications [188].
Early interventions with sitagliptin in patients with T2DM may have long-lasting reno- and islet-protective effects [189]. However, whether sitagliptin increases the risk of hospitalization in patients with HF remains debatable [190,191]. Sitagliptin decreased CVD incidence in patients with T2DM [192]. However, 12-week sitagliptin therapy did not reduce hepatic steatosis or fibrosis in overweight patients with T2DM [193]. Moreover, it did not reduce aminotransaminase levels in patients with NASH [194]. Vildagliptin exhibited a CVD risk comparable to sitagliptin [195], and it prevented the progression of T2DM-related CVD by improving LDL heterogeneity [196].
Position statement 11: Regressing hepatic steatosis/fibrosis and improving cardiovascular/metabolic outcomes are the optimal goals of pharmacological intervention for MAFLD.
MAFLD/CVD and other types of hepatitis
The prevalence of coexisting MAFLD and chronic hepatitis B (CHB) or chronic hepatitis C (CHC) is 30–70%, and MAFLD occurs in 13.6–59.3% of patients with CHB [197]. An inverse association has been reported between hepatitis B virus replication and hepatic steatosis [198], as fat deposition in hepatocytes and a related increasing inflammatory status may inhibit or suppress viral replication [199,200]. By contrast, patients with MAFLD and CHB tend to experience accelerated liver disease progression and exhibit more liver-related complications. Furthermore, their death rate is higher than that of patients with CHB or MAFLD [201]. More studies are warranted to explore the effect of coexisting CHB on CVD risk in patients with MAFLD.
Hepatic steatosis, a common histological feature, is detected in 30–70% of patients with CHC [202-204]. The coexistence of CHC and MAFLD occurs in 9–38% of cases [205]. Data suggest that metabolic disturbances are highly prevalent in patients with CHC, placing them at higher risks of CVD, carotid and coronary atherosclerosis, and myocardial dysfunction [206]. Nevertheless, no direct evidence suggests that MAFLD aggravates CVD risk in patients with CHC.
Delineating the relative contributions of alcohol consumption in patients with MAFLD having metabolic risk factors is challenging. Alcohol consumption may deteriorate liver disease and may lead to CVD development in patients with MAFLD through an additive or synergistic mechanism.
SUMMARY
MAFLD has become an important health issue globally. Because of underlying IR or metabolic derangement, substantial cross-talk occurs between hepatic outcomes (steatosis, a hepatic manifestation of metabolic syndrome) and cardiovascular events (CVD, a cardiac manifestation). In this positional statement, 11 important clinical issues regarding the diagnosis, screening, and assessment of MAFLD; the importance of the co-management of MAFLD and CVD; and potential management strategies have been addressed and discussed by both hepatologists and cardiologists. The benefits of various lifestyle modifications and updates on different pharmacological interventions for CVD and steatosis-associated advanced fibrosis have also been briefly reviewed. We hope that these statements simplify the clinical practice of gastroenterologists/hepatologists and cardiologists for treating patients with MAFLD or CVD. These statements also aim to draw the attention of general practitioners to emerging MAFLD, and setting optimal goals for clinical management is crucial.
Notes
Authors’ contributions
Conceptualization, CJL, YWW, and PNC; Writing, review, and editing the Original Draft, PNC; Review & Editing, WJC and CJYH; Writing and review: CLL, MLC, CCW, WTC, CYW, CYL, CLH, CYP, MLY, THC, J.FH, YHH, CYC, CEC, HCL, YHL, THL, JHK, TDW, and PYL.
Conflicts of Interest
Chern-En Chiang: I received honorarium from Astrazeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Menarini, MSD, Novartis, Novo Nordisk, Pfizer, Sanofi, Viatris.
The other authors declare no conflict of interests.
Acknowledgements
This work was supported by the National Science and Technology Council, Executive Yuen, Taiwan (MOST 109-2314-B-002 -091 -MY3; NSTC 112-2314-B-002 -205 -MY3).
This work was also partly supported by the “ Center of Excellence for Metabolic Associated Fatty Liver Disease, National Sun Yet-sen University, Kaohsiung, Taiwan” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, MOHW112-TDU-B-221-124007, NYCUKMU-111-I001 and NYCUKMU-111-I004, and by the Taiwan Association for the Study of the Liver.
Abbreviations
MAFLD
metabolic dysfunction-associated fatty liver disease
NAFLD
nonalcoholic fatty liver disease
T2DM
type 2 diabetes mellitus
HCC
hepatocellular carcinoma
CVD
cardiovascular diseases
IR
insulin resistance
NASH
nonalcoholic steatohepatitis
CAD
coronary arterial disease
VTE
venous thromboembolism
AST
aspartate aminotransferase
ALT
alanine aminotransferase
MRE
magnetic resonance elastography
FIB-4
fibrosis-4
LSM
liver stiffness measurement
MI
myocardial infarction
HF
heart failure
HfpEF
HF with preserved ejection fraction
LV
left ventricular
AF
atrial fibrillation
EASL
European Association for the Study of the Liver
APASL
Asian Pacific Association for the Study of the Liver
PPAR-γ
peroxisome proliferator-activated receptor gamma
EBMT
endoscopic bariatric and metabolic therapies
GLP-1RA
glucagon-like peptide-1 receptor agonist
DPP-4i
dipeptidyl peptidase-4 inhibitor
SGLT-2i
sodium-dependent glucose cotransporter-2 inhibitor
OCA
obeticholic acid
CHB
chronic hepatitis B
CHC
chronic hepatitis C

