Evidence-based hyponatremia management in liver disease
Article information
Abstract
Hyponatremia is primarily a water balance disorder associated with high morbidity and mortality. The pathophysiological mechanisms behind hyponatremia are multifactorial, and diagnosing and treating this disorder remains challenging. In this review, the classification, pathogenesis, and step-by-step management approaches for hyponatremia in patients with liver disease are described based on recent evidence. We summarize the five sequential steps of the traditional diagnostic approach: 1) confirm true hypotonic hyponatremia, 2) assess the severity of hyponatremia symptoms, 3) measure urine osmolality, 4) classify hyponatremia based on the urine sodium concentration and extracellular fluid status, and 5) rule out any coexisting endocrine disorder and renal failure. Distinct treatment strategies for hyponatremia in liver disease should be applied according to the symptoms, duration, and etiology of disease. Symptomatic hyponatremia requires immediate correction with 3% saline. Asymptomatic chronic hyponatremia in liver disease is prevalent and treatment plans should be individualized based on diagnosis. Treatment options for correcting hyponatremia in advanced liver disease may include water restriction; hypokalemia correction; and administration of vasopressin antagonists, albumin, and 3% saline. Safety concerns for patients with liver disease include a higher risk of osmotic demyelination syndrome.
INTRODUCTION
Hyponatremia is the most common electrolyte disturbance encountered in clinical practice and is associated with increased mortality and morbidity rates [1]. Liver disease, especially cirrhosis, is a relatively frequent etiology of hyponatremia. Hyponatremia in liver cirrhosis is typically defined as a serum sodium (sNa) concentration <135 mmol/L; however, some experts have defined hyponatremia as an sNa concentration <130 mmol/L [2,3]. The prevalence of sNa concentrations <135 mmol/L, <130 mmol/L, <125 mmol/L, and <120 mmol/L are 49.4%, 21.6%, 5.74%, and 1.2% in patients with liver cirrhosis, respectively [3]. Several studies have confirmed that hyponatremia in advanced liver cirrhosis is associated with poor outcomes, including refractory ascites (RA), hepatic encephalopathy (HE), hepatorenal syndrome (HRS), and mortality in advanced liver disease [4-6]. Given that sNa levels have been integrated into the model for the end-stage liver disease (MELD) score, which is used to determine liver transplantation (LT) priority, hyponatremia management is crucial [7].
Herein, we review the causes, factors, clinical features, and pathophysiology of hyponatremia in patients with liver disease. We also discuss the general treatment for hyponatremia in this patient population and management of hyponatremia before LT.
PATHOPHYSIOLOGY OF HYPOTONIC HYPONATREMIA IN LIVER DISEASE
The mechanisms of hyponatremia are mainly cirrhosis-induced hemodynamic compromise and other pathogenetic or superimposed factors.
Hypervolemic hyponatremia in patients with cirrhosis (primary mechanism) (Fig. 1)
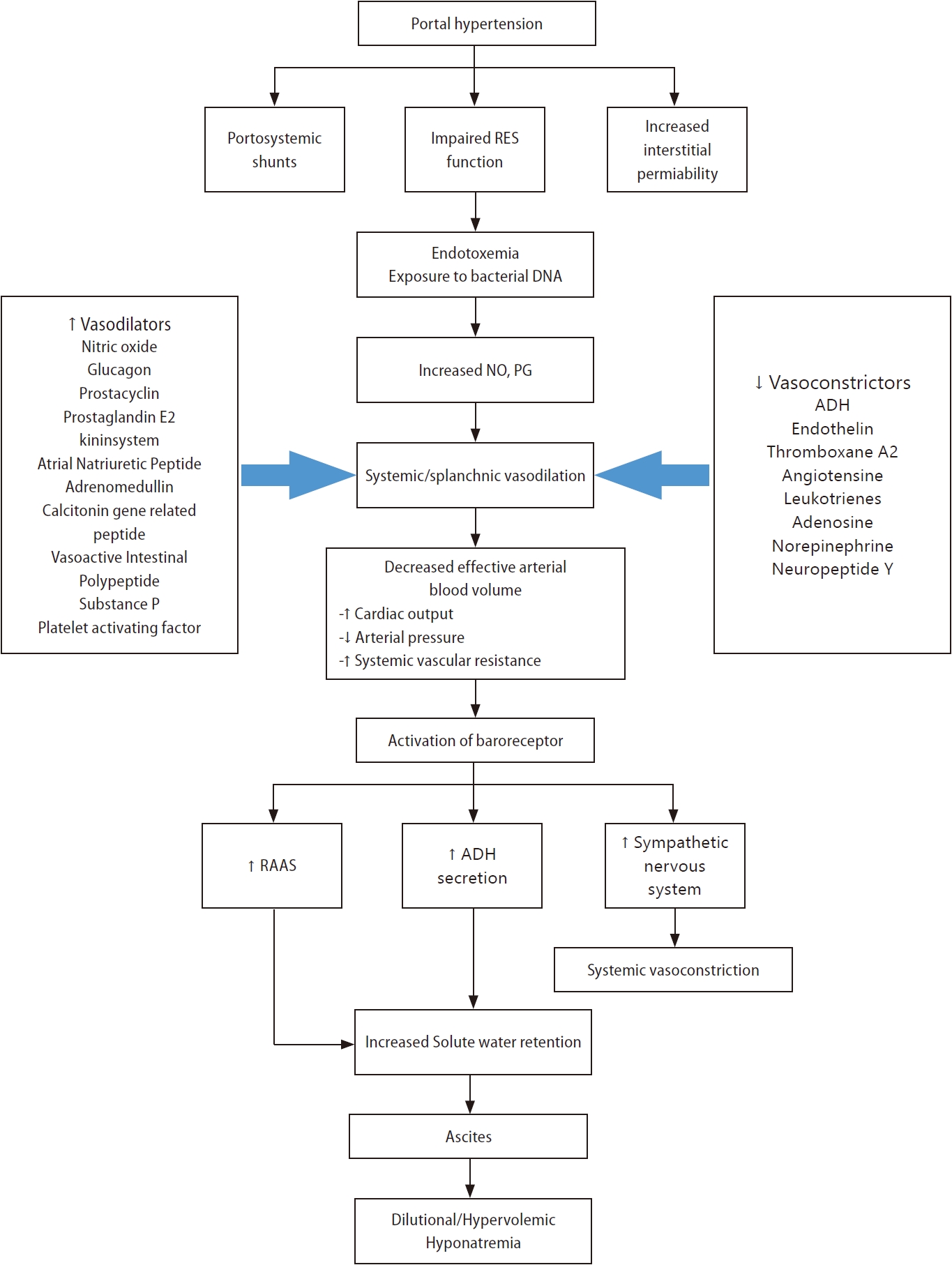
Pathogenesis of hypervolemic hyponatremia in patients with liver cirrhosis. ADH, antidiuretic hormone; DNA, deoxyribonucleic acid; NO, nitric oxide; PG, prostaglandin; RES, reticuloendothelial system.
Systemic or splanchnic vasodilation
The primary pathophysiologic mechanisms that lead to hypervolemic hyponatremia in patients with cirrhosis are systemic vasodilation and arterial underfilling [8]. Hyperdynamic circulation, resulting in increased cardiac output, which markedly reduces systemic vascular resistance and decreases mean arterial pressure, is common among patients with cirrhosis and progressive portal hypertension [9,10]. A significant decrease in vascular resistance is mainly associated with splanchnic vasodilation, owing to an increase in vasodilators and an opening of portosystemic collaterals [8,11]. Among vasodilators, the most important mediator is nitric oxide released by endothelial cells. Other vasodilators, such as glucagon, vasoactive intestinal peptide, substance P, platelet-activating factor, prostaglandins, prostacyclin, hydrogen sulfide, endocannabinoids, adrenomedullin, and endothelium-derived hyperpolarizing factors are involved in splanchnic vasodilation [12,13]. Vascular endothelial growth factors, tumor necrosis factor-alpha, mechanical stimuli due to shear stress, and decreased endotoxin or bacterial DNA clearance exist in the gastrointestinal tract due to dysfunction of the reticuloendothelial function and portal-systemic shunting [12,14,15]. Extrahepatic hyporeactivity to vasoconstrictors (endothelin-1, thromboxane A2, leukotrienes, norepinephrine, and antidiuretic hormone [ADH]) also contributes to systemic vasodilation, which is mediated by G protein-coupled transmembrane receptors [14].
Activation of endogenous vasoconstrictors (renin-angiotensin-aldosterone system, sympathetic nervous system, and ADH)
Systemic or splanchnic vasodilation and lack of arterial filling cause decreased carotid and renal baroreceptor pressure in patients with cirrhosis and portal hypertension [14]. As pressure is reduced due to a decrease in the effective circulation volume, sodium retention mechanisms, such as the renin-angiotensin-aldosterone system, sympathetic nervous system, and ADH, are activated to retain sodium and water [16-19]. Although these factors increase extracellular sodium stores, plasma volume, and cardiac output, the net effect is sodium and water reabsorption by the kidneys because the patient is substantially depleted of body fluid [15,20]. Serum osmolality is generally low in patients with decompensated cirrhosis; thus, ADH must be suppressed by osmotic stimulation [15]. However, non-osmotic stimuli act predominantly over osmotic stimuli to cause hyponatremia in patients with decompensated liver cirrhosis [15]. ADH released in response to hypovolemia in liver cirrhosis increases water permeability in the collecting ducts of the kidneys via aquaporin 2, a water channel protein [21-26]. This is one of the mechanisms leading to hypervolemic, dilutional hyponatremia in liver cirrhosis [14,15].
Other causes of hypotonic hyponatremia in patients with liver disease
Hypervolemic hyponatremia
Kidney failure related to end-stage liver disease or acute kidney injury, including HRS, impairs urine dilution and causes dilutional hyponatremia accompanied by water retention [1,27-29]. Nephrotic syndrome due to hepatitis B or C viral infection causes hyponatremia [27,30,31]. Coexistent cardiac disorders, including heart failure (HF) or cardiomyopathy (e.g., alcoholic or hemochromatosis) in patients with liver disease, may result in hyponatremia via neurohormonal mechanisms [27,32].
Hypovolemic hyponatremia
Depletion of effective arterial volume via non-renal sodium loss (lactulose-associated diarrhea or vomiting), renal sodium loss (diuretics such as spironolactone/eplerenone), or primary adrenal insufficiency (autoimmune adrenalitis in autoimmune hepatitis or adrenal tuberculosis in alcoholics) can markedly increase secondary ADH release leading to water retention [1,27,33].
Euvolemic hyponatremia
Syndrome of inappropriate antidiuresis (SIAD) may be caused by infection or medications used in the treatment of liver disease (terlipressin [34-36], interferon, brivanib [37], sorafenib [37], cixutumumab [38], and boceprevir [39]), acute intermittent porphyria, and malignancy (hepatocellular carcinoma) [27]. Endocrine disorders, including secondary adrenal insufficiency (caused by the pituitary gland or hypothalamus) or severe hypothyroidism, are rare causes of hyponatremia in patients with liver disease.
CLASSIFICATION OF HYPONATREMIA
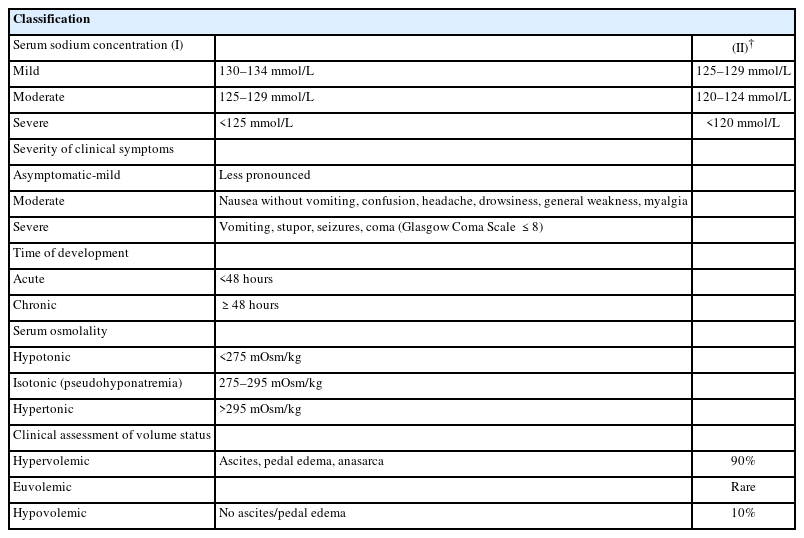
The accurate classification of hyponatremia in patients with liver disease is crucial for appropriate diagnosis and management. Hyponatremia is commonly classified based on the sNa concentration, severity of clinical symptoms, duration, and extracellular fluid (ECF) status (Table 1).
Classification of hyponatremia based on the severity of clinical symptoms and duration
According to the severity of clinical symptoms, hyponatremia can be divided into either moderate (nausea, confusion, and headache) or severe/profound (vomiting, seizure, cardiopulmonary collapse, and coma). Based on the duration, hyponatremia can be differentiated as acute (<48 h) or chronic (>48 h) [1]. Patients with acute hyponatremia develop neurological symptoms due to cerebral edema because they do not have enough time to develop mechanisms that can prevent cerebral edema [40]. However, most patients with advanced cirrhosis have chronic hyponatremia, which allows for astrocytes to adapt and thus symptoms may not appear, making a diagnosis based on symptoms difficult [15].
Classification of hyponatremia based on volume status
Hyponatremia is classified into three types according to their ECF status: hypervolemic, euvolemic, and hypovolemic hyponatremia [41-43]. Patients with liver cirrhosis commonly have hypervolemia (increased ECF volume), characterized by ascites, anasarca, and/or pedal edema [2,15,20]. Hypervolemia is caused by sodium retention with impaired free-water excretion, resulting from renin-angiotensin-aldosterone or sympathetic nervous system activity and ADH hypersecretion, leading to dilutional hyponatremia [2,15]. Euvolemic hyponatremia is rare except when with SIAD or endocrine disorders, including adrenal insufficiency and severe hypothyroidism. Some patients (<10%) with liver cirrhosis have hypovolemic hyponatremia due to ECF loss after diuretic use or diarrhea/vomiting, characterized by a lack of ascites and pedal edema [44].
DIAGNOSTIC APPROACH TO HYPONATREMIA
Based on a comprehensive assessment of the history (recent body fluid loss, high water intake with low salt, presence of a malignant tumor, recent surgery, and use of drugs [thiazide diuretics, intravenous immune globulin, and terlipressin], physical examination (detailed neurologic evaluation and assessment of ECF volume), and laboratory findings (serum and urine osmolality and urine sodium), the physician can determine the underlying cause of hyponatremia and adjust the treatment accordingly for patients with liver disease [45-47].
Steps for hyponatremia diagnosis
A practical diagnostic approach to hyponatremia, which is similar to that used for the general population, is utilized for patients with liver disease. The step-by-step process is presented below (Fig. 2).

Hyponatremia diagnostic approach in patients with liver disease. CHF, congestive heart failure; GI, gastrointestinal; LVP, large volume paracentesis; SIAD, syndrome of inappropriate antidiuresis.
Step 1. Rule out pseudohyponatremia and calculate the glucose-corrected sNa concentration in patients with hyperglycemia
The main objective of this step is to determine whether the hyponatremia is hypotonic. The serum osmolality is measured to differentiate hypotonicity [1]. For serum osmolality that is <275 mOsm/kg (hypotonic hyponatremia), further predefined steps are followed, while for serum osmolality that is ≥275 mOsm/kg, hyponatremia may be hypertonic (usually from hyperglycemia) or isotonic (due to pseudohyponatremia). In patients with hyperglycemia, the corrected sNa concentrations are calculated based on equations (1) and (2) [48,49]:
Pseudohyponatremia is defined as a spurious decrease in plasma sodium (<135 mmol/L) due to marked hyperproteinemia or hyperlipidemia [1]. Hyperproteinemia with increased globulin due to autoimmune hepatitis (elevated immunoglobulin G), primary biliary cirrhosis, alcoholic liver disease (elevated immunoglobulin M and immunoglobulin A), or liver cirrhosis (elevated γ-globulin) are occasionally observed in patients with chronic liver disease [27]. Moreover, marked dyslipidemia is often observed in patients with liver disease. Hypertriglyceridemia can occur due to excessive alcohol consumption, interferon treatment, and uncontrolled diabetes mellitus; mixed dyslipidemia can occur due to hepatitis B virus- or C virus-related nephrotic syndrome; and hypercholesterolemia can occur due to primary biliary cirrhosis or other cholestatic liver diseases [50-53]. Consequently, the aforementioned liver diseases are potential causes of pseudohyponatremia. Pseudohyponatremia is commonly observed using indirect ion-selective electrodes for serum electrolyte measurement [19,54]. Therefore, the use of direct ion-selective electrodes or an osmometer for serum osmolality measurements is suggested to determine the true sNa concentration [19,54].
Step 2. Evaluate the severity of clinical hyponatremia symptoms
Symptomatic moderate or severe/profound hyponatremia should be treated promptly with hypertonic saline to improve symptoms, and this should be prioritized over further diagnostic testing [1,55]. If acute management has been initiated or there is asymptomatic or mild hyponatremia, step 3 should be followed.
Step 3. Measure urinary osmolality
When urinary osmolality falls to 100 mOsm/kg or below, which indicates maximally diluted urine, excessive water and hypotonic food or fluid (e.g., beer, rice wine, liquid diet) should be discontinued [1,55].
Step 4. Classify hyponatremia based on urine sodium concentration and extracellular fluid status
The ECF status is divided into hypovolemia, euvolemia, or hypervolemia using the patient’s history and physical examinations. A urine sodium level ≤30 mmol/L indicates low effective arterial volume, including hypovolemia and hypervolemia. For contracted ECF volume (hypovolemia), non-renal sodium loss including diarrhea, vomiting, or remote diuretics should be considered, while for expanded ECF volume (hypervolemia), liver cirrhosis, HF, and nephrotic syndrome should be considered. When the urine sodium level is >30 mmol/L, diuretics and kidney disease should be ruled out and ECF status should be assessed. For contracted ECF volume (hypovolemia), diuretics or renal sodium loss including renal or cerebral salt wasting should be considered, while for normal ECF volume (euvolemia), SIAD, secondary adrenal insufficiency, or hypothyroidism should be ruled out [1,27,55]. The clinical assessment to determine the volume status is difficult as both the sensitivity (0.5–0.8) and specificity (0.3–0.5) are low [1]. Therefore, additional modalities such as fractional excretion of sodium (with a cutoff of 1) or urea (with a cutoff of 35) or point-of-care ultrasound (POCUS) can be helpful for assessing volume status [56,57]. The fractional excretion of urea can provide additional assistance in patients receiving diuretics [57].
Step 5. Conduct a rapid adrenocorticotropic hormone test, assess thyroid function, and measure serum creatinine
Coexisting factors, including adrenal insufficiency, severe hypothyroidism, and kidney failure, should be evaluated.
CLINICAL SIGNIFICANCE OF HYPONATREMIA IN LIVER DISEASE
Hyponatremia has been linked to higher morbidity and mortality in patients with acute and chronic liver disease, acute-on-chronic liver failure (ACLF), or those awaiting LT [58,59].
Liver cirrhosis
Several studies have found that hyponatremia in liver cirrhosis is associated with a high incidence of liver-related complications, including a high prevalence of RA, HE, spontaneous bacterial peritonitis, and HRS; an increased need for massive paracentesis; and a shorter interval between paracenteses [3].
Hepatic encephalopathy and hyponatremia
Patients with acute hyponatremia have a higher incidence of neurological symptoms (such as headache, disorientation, confusion, focal neurological deficits, seizures, and in some cases, death due to brain herniation) than patients with chronic hyponatremia [60]. Hypoosmolar hyponatremia expands astrocytes by moving water from the extracellular space into astrocytes to maintain osmotic balance; however, the expansion of brain cells is constricted due to the limited anatomical space [60,61]. Accordingly, intracellular solutes are discharged in the opposite direction to reduce intracellular osmotic pressure. Intracellular potassium is excreted first, followed by organic osmotic pressure, including myoinositol, glutamine, choline, and taurine shifts [60,61]. This protective adaptation mechanism can be observed in chronic hyponatremia and liver disease. The secondary increase in the intracellular glutamine content due to ammonia metabolism acts synergistically, mainly resulting in astrocyte edema and dysfunction of the glial-neuronal communication pathway [15,62,63]. Hyponatremia then further exacerbates astrocyte swelling and causes HE in patients with liver cirrhosis [4,15]. Hyponatremia is a major risk factor for HE in patients with liver cirrhosis, increasing HE risk by 8.36 times [4]. Therefore, monitoring sNa levels in patients with cirrhosis is critical for preventing or managing HE.
Refractory ascites, acute kidney injury, and hepatorenal syndrome
The pathogenesis of ascites is similar to that of hyponatremia due to excess water in the body, arterial vasodilation, arterial underfilling, and activation of the renin-angiotensin-aldosterone system and ADH [64-66]. In addition, a large amount of body fluid may accumulate due to increased intestinal capillary permeability resulting from the decrease in albumin synthesis caused by decreased liver function [66]. As much as 10% of patients with decompensated liver cirrhosis develop RA, characterized by severe ascites and requiring repeated large-volume paracenteses, even after receiving adequate diuretics and a salt-restricted diet [64-67]. One study found the incidence of RA to be higher in patients with hyponatremia than in patients with normal concentrations of sNa (sNa level <130 mmol/L: 29.4% vs. sNa level >135 mmol/L: 13.5%, p<0.001) [3]. A study of American veterans showed that the 1-year mortality rate for patients with RA was close to 70% [68]. Similarly, a study in Spain showed that the 1-year survival rate among patients with RA was only 31.6% and also showed that hyponatremia was an independent predictor of survival in patients with cirrhosis and RA [69].
Approximately 14–50% of patients with ascites and hyponatremia develop renal dysfunction in the form of acute renal injury or HRS [44,69,70]. Renal failure is more common in patient s who have hy p onatremia with ascites, and hyponatremia is known to be an independent risk factor for acute renal injury (28% of those with sNa levels >135 mmol/L, 40% of those with sNa levels <130 mmol/L) [3].
Acute-on-hronic liver failure
ACLF is defined as acute and rapidly progressive liver failure in patients with previously diagnosed or undiagnosed chronic liver disease, associated with multi-organ failure and very high short-term mortality [59,71]. Approximately 25% of patients with ACLF have hyponatremia [59], and the frequency of hyponatremia is higher on the first day of hospitalization in patients with acutely decompensated cirrhosis and ACLF (without ACLF: 10.5% vs. with ACLF: 22.2%) [71]. In cirrhotic patients hospitalized with bacterial infection, the presence of hyponatremia is an independent predictor of ACLF and subsequent mortality [72].
Liver transplantation
A prospective cohort study showed that patients referred for consideration of LT with hyponatremia have a higher risk of early death regardless of cirrhosis severity, as assessed using the MELD score [5]. Therefore, some researchers have advocated for rapid LT in patients with cirrhosis and a MELD score <21, persistent ascites, and hyponatremia [5]. Another prospective multicenter study showed that incorporating the sNa level into the MELD score more accurately predicted survival compared to the MELD alone [73]. As a result of these studies, the United Network for Organ Sharing incorporated the sNa level into the MELD score (MELD-Na) for organ allocation in January 2016 [74].
The effect of pre-transplant hyponatremia on survival after transplantation is controversial. The largest study (n=19,537) conducted during the MELD era showed no significant reduction in survival after LT for patients with hyponatremia [75]. In another study conducted in the US, no significant difference in the survival rate at 90 days after LT between recipients with normal sNa concentrations and those with hyponatremia was found [76]. Another UK study revealed that the 3-year risk-adjusted mortality rate was higher in patients with pre-transplant hyponatremia than those with normal sNa levels [77]. A small single-center study also showed a relative decrease in 3-month survival in patients with an sNa concentration <130 mmol/L compared to patients without hyponatremia [78]. In addition, patients with hyponatremia had a high incidence of neurologic complications, renal failure, prolonged hospitalization in an intensive care unit, and increased ventilator requirements after LT [78]. The impact of pretransplant hyponatremia on posttransplant prognosis is still controversial; however, hyponatremia correction before transplantation is one of the methods used to reduce the risk of various complications.
Osmotic demyelination syndrome
Osmotic demyelination syndrome (ODS) is a rare but irreversible neurologic complication from excessive hyponatremia therapy that can result in death. When sNa levels return to normal, electrolytes and osmolality are restored in brain cells. Electrolytes are rapidly corrected, whereas organic osmolality is corrected slowly. This results in the shrinkage of glial cells, axonal shear damage, tight junction destruction, and cell death [79]. ODS occurrence is difficult to predict [80]; however, it has been established that individuals who are alcoholics, malnourished, or have advanced liver disease and are liver transplant recipients are at high risk for developing ODS, especially during overcorrection of chronic hyponatremia [27,81]. Furthermore, the conditions that increase susceptibility to ODS, including malnutrition, hypokalemia, and hypoxia, are frequently observed in patients with liver disease [27,81]. ODS symptoms can range from mild behavioral changes to dysarthria, dysphagia, ataxia, Parkinson’s disease, and widespread neurological deficits. According to the 2013 American guidelines, patients with advanced liver disease are classified as high risk for developing ODS; thus, a lower target of 4–6 mmol/L/day, with a maximum of 8 mmol/L in any 24-hour period, is recommended [81]. Therefore, assessing the underlying disease and major risk factors for ODS is critical for the proper management of hyponatremia in patients with liver disease.
RECOMMENDATION OF MANAGEMENT
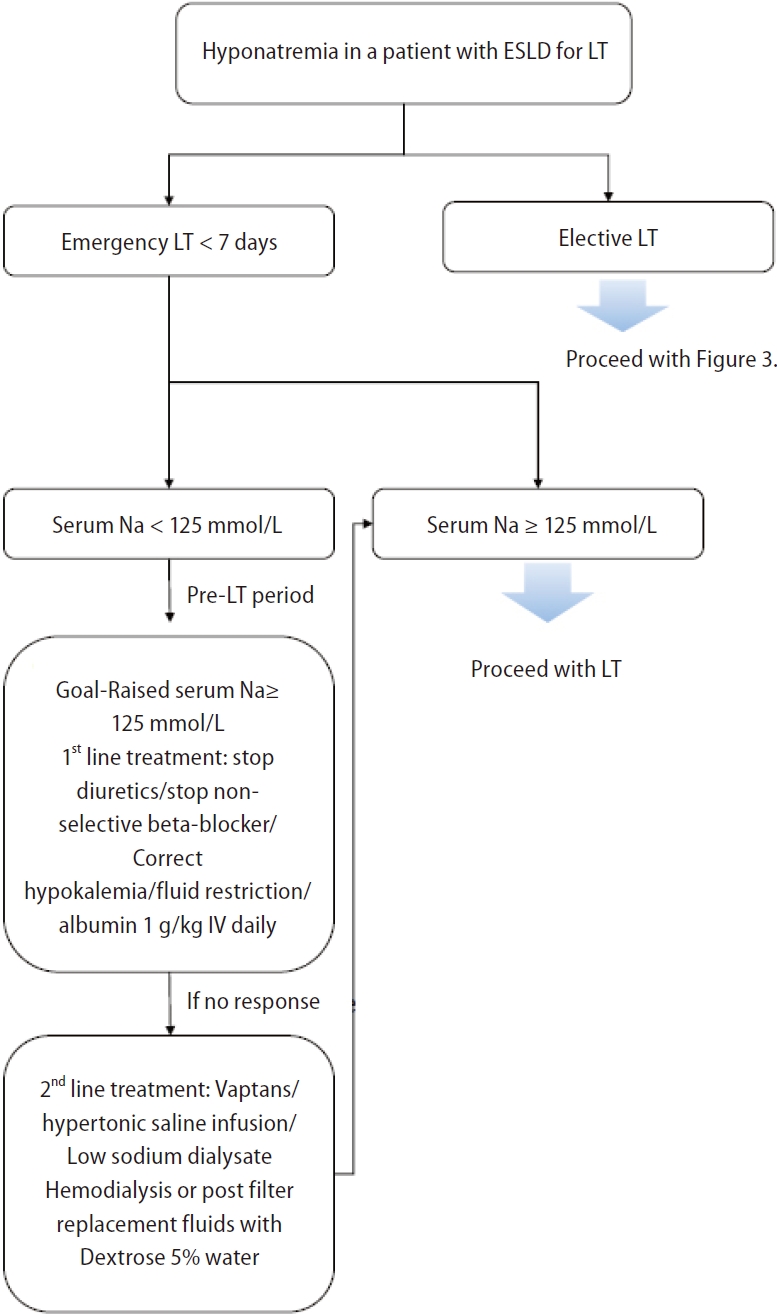
The approach to hyponatremia treatment in liver disease is similar to that used in the general population; however, some specific considerations are required in advanced liver disease. Hyponatremia management is guided by the severity of symptoms (asymptomatic or symptomatic [mild, moderate, severe]) and duration of hyponatremia (acute [<48 h] or chronic [≥48 h or unknown]). Hyponatremia treatment aims to prevent a further decrease in the sNa concentration, reduce intracranial pressure in patients at risk of brain prolapse, relieve hyponatremia symptoms, and prevent overcorrection. Hyponatremia management strategies in patients with liver disease are presented in Figure 3.

Treatment of hyponatremia in patients with liver disease. GCS, Glasgow Coma Scale; OLT, orthotopic liver transplant; sNa, serum sodium concentration. *Acute, If the hyponatremia has developed over a period of less than 48 hours. †Chronic, If the hyponatremia has been present for 48 hours or more or if the duration is unknown.
Symptomatic acute or chronic hyponatremia
The 2013 American guidelines [81] and 2014 European guidelines [1] recommend that patients with moderately or severely symptomatic hyponatremia undergo treatment with hypertonic saline. In cases of severe symptomatic hyponatremia, rapid intermittent bolus regimens of hypertonic saline should be immediately infused to raise sNa levels by 4 to 6 mmol/L to improve cerebral edema. In cases of moderate symptomatic hyponatremia, rapid intermittent boluses or slow continuous infusions of hypertonic saline may be administered. The target correction rate is an sNa concentration of 5–9 mmol/L within the first 24 hours and 10–17 mmol/L or 130 mmol/L within 48 hours [82]. However, for patients with decompensated liver cirrhosis, administration of hypertonic saline should be limited to severely symptomatic patients with life-threatening conditions such as cardio-respiratory distress, seizures, and coma because it may worsen volume overload or increases ascites and edema. Hypertonic saline is also used to increase sNa levels in patients with hyponatremia before LT to prevent rapid hyponatremia correction after LT [83]. The correction rate for hyponatremia should not exceed 8 mmol/L in any 24-h period in advanced liver disease because of the high risk for ODS [81].
Asymptomatic acute hyponatremia
An absence of symptoms indicates that significant brain swelling has not yet occurred. Therefore, instead of administering hypertonic saline infusions, the etiology of hyponatremia must be assessed and cause-specific treatments be initiated. However, if the acute drop in sNa concentration exceeds 10 mmol/L, hypertonic saline should be administered to prevent a further decrease in the concentration [1]. Hypertonic saline administration may be delayed if hyponatremia is corrected by water diuresis. Urine volume, low urine specific gravity, and low urine osmolality can help clinicians determine the hypertonic saline infusion volume. The treatment goal is to rapidly increase the sNa concentration to 4–6 mmol/L to relieve symptoms and prevent brain hernias [1].
Asymptomatic chronic hyponatremia
Asymptomatic chronic hyponatremia does not require urgent correction; however, identifying and treating the underlying cause is critical. In liver cirrhosis, hyponatremia is mostly the chronic asymptomatic type due to brain adaptation. Therefore, it is essential to differentiate the cause of hyponatremia based on the ECF volume status.
Hypovolemic hyponatremia is caused by excessive use of diuretics and reduced effective volume due to gastrointestinal bleeding. Thus, hypovolemic hyponatremia must be corrected by discontinuing the diuretics and expanding the plasma volume using crystalloid fluids, albumin, etc.
Hypervolemic or dilutive hyponatremia is common and worsens as liver disease advances. Considering the etiology of hypervolemic hyponatremia, the ideal solution is to increase the excretion of water without solutes. Various management methods have been introduced and are discussed below. The treatment evidence is summarized in Table 2.
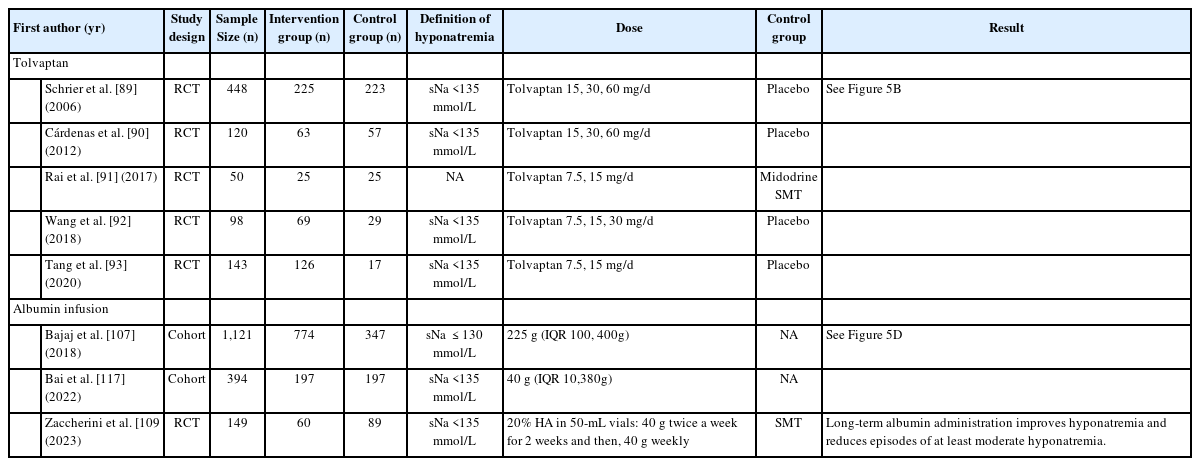
Characteristics of the studied for the treatment of hyponatremia in liver disease according to use of tolvaptan or albumin infusion
Water restriction
Fluid restriction has been suggested as a first-line treatment for hyponatremia. For effective fluid restriction, water intake should be about 500 mL/day less than the sum of urine output and insensible loss (1–1.5 L/day) [14]. Fluid restriction should be considered if there are neurological symptoms or if the sNa concentration is <120 mmol/L; however, the role of fluid restriction is limited in patients with mild, asymptomatic hyponatremia [3]. The water restriction method is associated with poor patient compliance, making it difficult to achieve sNa normalization. A good indicator of adequate fluid restriction is a change in the sNa concentration within the first 24–48 hours. However, if sNa levels do not increase within the first 48–72 hours, other options should be considered [84].
Correction of hypokalemia
Hypokalemia may result from increased urine loss due to diuretic treatment or increased gastrointestinal fluid loss in patients with diarrhea or vomiting. Hypokalemia correction is recommended for two reasons: hypokalemia increases renal ammonia synthesis, and concomitant alkalosis increases the fraction of bound ammonia in plasma, which can lead to HE. When osmotically active potassium is corrected, replenished potassium enters the cells, and intracellular sodium moves in the opposite direction, increasing the sNa concentration and osmotic pressure without external sodium administration [45,47]. Hence, since potassium is as osmotically active as sodium, excessive sNa and hypokalemia corrections can cause severe complications such as ODS due to overcorrection [45].
Vasopressin receptor antagonists or vaptans
Key question: In hyponatremic patients with liver disease, does the vaptan group show more effective sodium correction than the no vaptan group?
Vasopressin receptor antagonists or vaptans are non-peptide drugs that inhibit ADH action on the V1a, V1b, and V2 receptors [85]. The V2 receptor responsible for water absorption is located in the basolateral membrane of the renal collecting duct, and a blockade of this receptor can cause water diuresis and increase the sNa concentration (Fig. 4) [86]. The V2 selective blocker of ADH effectively improves the sNa concentration in SIAD or HF cases with elevated vasopressin [87].
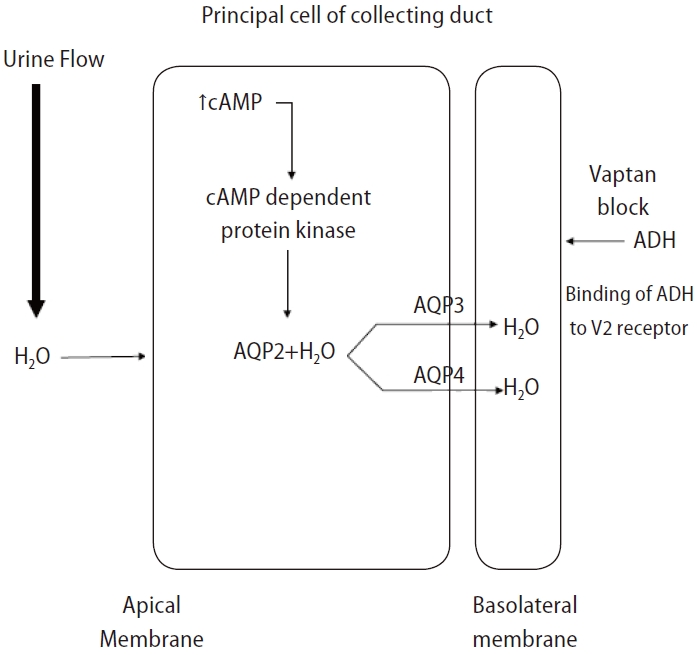
Mechanism of action of vaptan. ADH, antidiuretic hormone; AQP2, aquaporin-2; AQP3, aquaporin-3; AQP4, aquaporin-4.
Tolvaptan is the only selective V2 receptor antagonist that can be administered orally, and several studies have shown that tolvaptan significantly increases the sNa concentration and improves ascites in patients with liver cirrhosis, but does not improve the survival rate. In a multicenter clinical trial that followed patients with HF, SIAD, and liver cirrhosis longitudinally for an average of 1.9 years, the sNa concentration significantly increased in patients with HF, SIAD, and liver cirrhosis after taking tolvaptan; however, the increase in the sNa concentration in patients with liver cirrhosis was slower than that in patients with HF and SIAD [88]. To evaluate the effect of tolvaptan in terms of sodium correction, we performed a database search of Ovid MEDLINE and conducted a meta-analysis of existing randomized controlled trials (Fig. 5A). Tolvaptan was found to be effective at correcting hyponatremia in patients with liver disease (Fig. 5B and Table 2) [89-93]. Additionally, the beneficial effect of vaptan on ascites was confirmed in three meta-analyses [94-96]. A recent cohort study showed that an increase in sNa levels after one month of tolvaptan treatment could positively affect the risk of death in patients with cirrhosis and hyponatremia [97]. However, a previous meta-analyses reported no change in the short-term and long-term survival of patients with cirrhosis after treatment with vaptan [94-96]. For patients with cirrhosis and hyponatremia at baseline, survival rates tended to improve after treatment with vaptan, though it was not statistically significant [96]. The most common side effects of tolvaptan include thirst, polyuria, and dry mouth. Serious side effects such as dehydration with hypotension, dehydration with dizziness, syncope, and acute renal failure can also occur. In an open-label phase 4 study aimed at evaluating the efficacy and safety of tolvaptan in autosomal dominant polycystic kidney disease, aspartate transaminase and alanine transaminase levels were elevated in two patients (1.7%), and these abnormal findings improved after maintaining the dose or temporarily discontinuing tolvaptan [98]. Therefore, the US Food & Drug Administration has limited tolvaptan use to less than 30 days and recommends that it not be used in patients with underlying cirrhosis due to the risk of liver failure and death [99]. However, it should be noted that the dose of tolvaptan in this study was 4–8 times the dose commonly used to treat hyponatremia [100]. The European Association for the Study of the Liver clinical practice guidelines for decompensated cirrhosis recommends the routine use of tolvaptan for correction of low sodium levels only in controlled clinical trials [101]. Korea’s Ministry of Food and Drug Safety has approved tolvaptan for hyponatremic patients with HF or SIAD, but not for patients with liver disease [102]. The Japanese Society of Gastroenterology does not mention tolvaptan use for correcting hyponatremia; however, tolvaptan has been approved as an additional drug for fluid retention in patients with liver cirrhosis and is recommended as an additional drug for patients with ascites that is refractory to treatment with existing diuretics (furosemide and spironolactone) [103]. The use of vaptans in patients with reduced liver function is restricted in most countries, and caution is advised when administering vaptans because relevant studies on long-term survival are lacking.
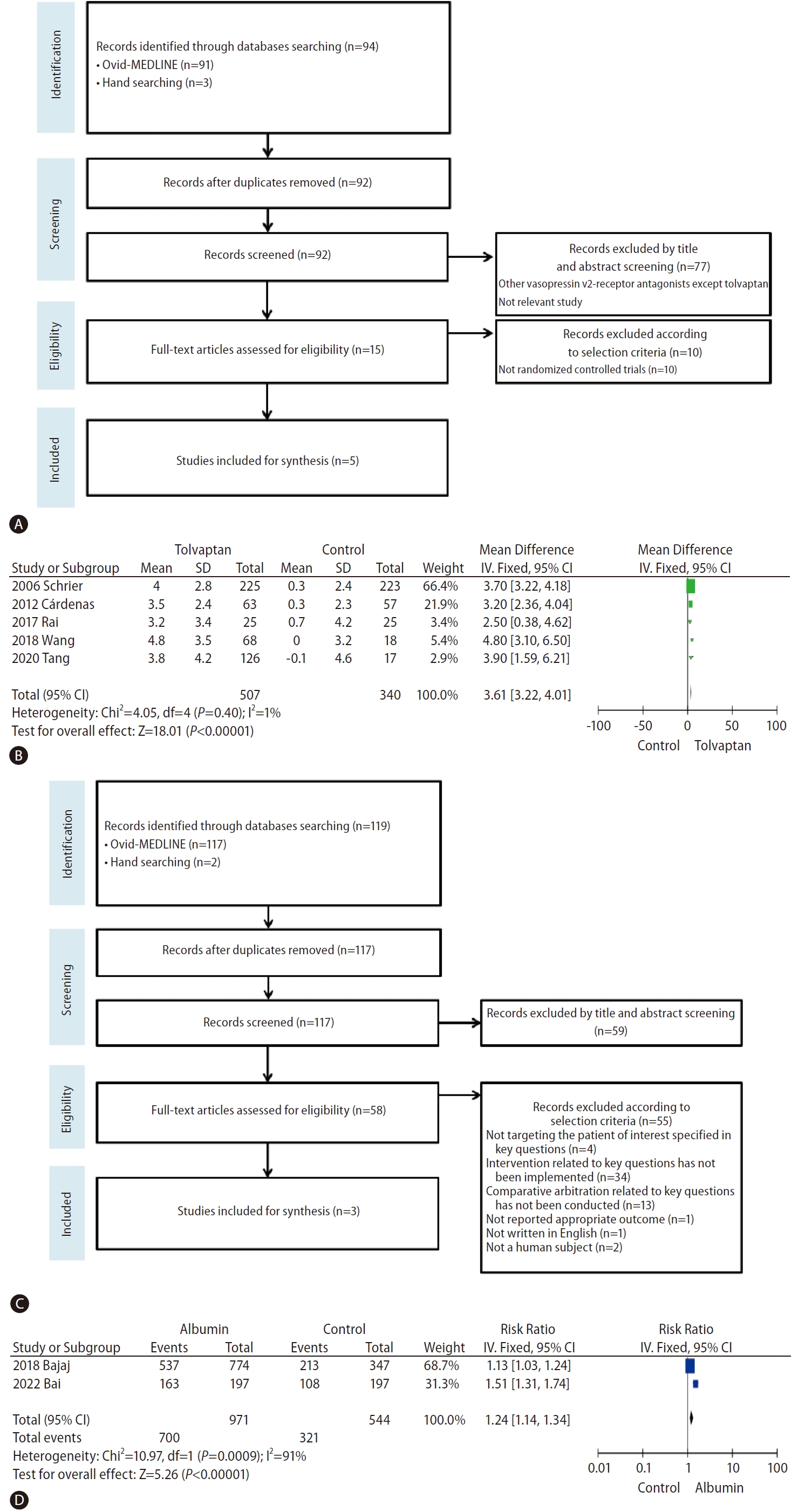
Results of hyponatremia treatment in liver disease. (A) Flowchart of selection of studies on the effect of tolvaptan treatment on hyponatremia. (B) Forest plot showing the effect of tolvaptan treatment on hyponatremia (change from baseline hyponatremia [mmol/L]). (C) Flowchart of selection of studies on the effect of albumin treatment on hyponatremia. (D) Forest plot showing the effect of albumin treatment on hyponatremia (improvement in hyponatremia [%]).
Albumin
Key question: In hyponatremic patients with liver disease, does the albumin group show more effective sodium correction than the no albumin group?
Albumin replacement is another treatment option that can improve hyponatremia associated with liver cirrhosis. Furthermore, albumin replacement in patients with cirrhosis has been widely used to manage various complications such as spontaneous bacterial peritonitis, HRS, ascites, and HE [104-109]. Previous authors have argued that intravenous albumin infusions can increase the sNa concentration through expanding the intravascular volume, thus increasing free water clearance in the urine [102,110]. Regarding the pathogenesis of hyponatremia in liver cirrhosis, the sNa concentration can be increased by maintaining colloidal osmotic pressure and affecting the inflammatory pathway, improving the hypervolemic circulation state, and removing inflammatory factors [111].
Most previous studies have evaluated the role that albumin replacement plays in preventing hyponatremia after largevolume paracentesis in cirrhosis hyponatremia, but relatively few studies have evaluated the role that albumin plays in hyponatremia treatment [112-117]. In a retrospective multicenter study of 1,125 patients with liver cirrhosis accompanied by hyponatremia (regardless of large-volume paracentesis) at the time of admission, the delta values of the sNa concentrations between the albumin replacement group and the nonalbumin replacement group were compared, showing significantly higher delta values in the albumin replacement group [113]. In addition, the post-hoc analysis of the ANSWER study, which compared the group receiving long-term albumin treatment (20% human albumin in a 50 mL vial: 40 g twice a week for 2 weeks, then 40 g weekly thereafter) to the group receiving standard medical treatment, showed at the 3-month follow-up that albumin was associated with sNa normalization over time [115]. The most recent meta-analysis evaluating the efficacy of albumin replacement also suggested that albumin replacement can be helpful in the prevention and treatment of hyponatremia in liver cirrhosis [116]. However, recommendations on the use of albumin replacement therapy in the management of cirrhosis with hyponatremia vary according to clinical guideline. While some suggest that albumin should be used to prevent or treat hyponatremia in liver cirrhosis [103], other reports recommend that albumin infusions be carefully evaluated due to lack of data [111,118].
We searched the Ovid MEDLINE database for studies evaluating the efficacy of albumin infusions for sodium correction in patients with liver disease. Only one RCT and two cohort studies were found that evaluated the use of albumin in the treatment of hyponatremia for patients with liver cirrhosis. The results of this one RCT are replicated here, and we performed a meta-analysis of the two cohort studies (Fig. 5C and Table 2) [113,115,117]. Our analysis (Fig. 5D) showed that the use of albumin was significant for treating hyponatremia that had already occurred. In most studies, the use of albumin resulted in significant improvements in hyponatremia; however, longterm outcomes, including the survival rate, showed a heterogeneous relationship. In a cohort study conducted by Bajaj et al. [113] (2018) that evaluated 1,126 patients with hyponatremia, the albumin group showed resolution of hyponatremia and a higher 30-day survival rate. However, a post hoc analysis of the ATTIRE trial conducted in 2021, which included 206 hyponatremic patients, showed that albumin infusions were not associated with mortality [114]. In another cohort study conducted by Bai et al. [117] (2022), which included 339 patients with liver cirrhosis, albumin infusions were found to effectively improve serum sodium levels during hospitalization in patients with cirrhosis and hyponatremia, but did not have a significant effect on long-term mortality.
Although high-quality studies on hyponatremia treatment are lacking, it appears that albumin injections may help prevent and treat hyponatremia in patients with cirrhosis. However, these findings must be validated through a well-designed, large-scale study, and the optimal dosages and durations of treatment and their effect on outcomes, including survival rates, must be further evaluated.
Perioperative management of patients awaiting liver transplantation
Pre-transplant hyponatremia is associated with prolonged hospitalization, prolonged intensive care unit admission, and neurologic complications [76-78,119,120]. Efforts to optimize sNa levels to >125 mmol/L before LT are needed, as patients with severe hyponatremia before transplantation are more prone to neurological complications such as ODS after transplantation [76,121,122]. The risk of ODS after LT in patients with liver disease increases with lower baseline sNa levels [76]. Patients undergoing LT are at increased risk for rapid sNa correction due to fluid shifts that occur during surgery from the intraoperative administration of intravenous crystalloids, blood products, and sodium bicarbonate [10,83,123]. However, despite the importance of correcting hyponatremia, no definitive protocol for perioperative management of hyponatremia and no correction threshold for sNa levels are currently available [123].
The incidence of ODS, a severe complication caused by rapid sNa correction after LT, ranges from 0.5% to 29% [7,76,124]. Symptoms such as quadriplegia (up to 45%) and seizures (27–36%) occur within 1–2 weeks after LT [7,125,126]. Since ODS is associated with poor prognosis [76,125,126], various methods have been used to prevent rapid correction of sNa. LT recipients receive a large amount of sodium-containing intravenous fluids, such as packed red blood cells, platelet concentrates, and fresh frozen plasma; thus rapid sNa correction may occur after surgery [10,83,123]. To minimize this rapid correction, 0.45% saline or 5% glucose water can be used instead of 0.9% normal saline [127]. Additionally, prothrombin complex concentrates with low sNa levels can be used instead of fresh frozen plasma [127]. Finally, for patients who have received or will receive high intraoperative sodium loads through blood transfusions and intravenous fluids, continuous veno-venous hemofiltration using dilute solutions before or during surgery may be considered for managing rapid changes in sNa levels [123]; however, relevant data are very limited.
Since hyponatremia improves when fundamental liver function is restored after LT, the occurrence of severe complications such as ODS due to rapid changes in sNa must be monitored. Some studies have shown that mortality due to ODS after LT is 40% at 3 months [126] and 63% at 1 year [76], and up to 84% of surviving patients have been reported to suffer from permanent sequelae [125,126]. Desmopressin administration as a rescue measure is useful when unintended overcorrection of hyponatremia occurs [128]. One proposed approach for the perioperative management of hyponatremia in patients awaiting LT is summarized in Figure 6 [129].
CONCLUSION
Hyponatremia is a pervasive electrolyte disorder in advanced chronic liver disease. Fundamental treatment strategies include water restriction, hypokalemia correction, and diuretic discontinuation. Hypertonic saline can be considered in acutely symptomatic hyponatremia; however, caution is advised given the potential for fatal complications such as ODS and worsening ascites. Furthermore, although vasopressin receptor antagonists and albumin replacement can be used to correct hyponatremia in liver cirrhosis, supportive studies are lacking, and individual countries may have controversies regarding their use. Although LT is ideal for managing hyponatremia in patients with advanced chronic liver disease, multidisciplinary approaches to perioperative management are essential for correcting hyponatremia.
Notes
Authors’ contribution
JYR and SHB contributed to manuscript research and writing. SHB and SK contributed to the conceptualization and critical review and editing of the manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This study was supported by a grant from the National Research Foundation of Korea (no. 2021R1C1C1008966).
Abbreviations
ADH
antidiuretic hormone
ECF
extracellular fluid
HF
heart failure
HE
hepatic encephalopathy
HRS
hepatorenal syndrome
LT
liver transplantation
MELD
model for end-stage liver disease
ODS
osmotic demyelination syndrome
RA
refractory ascites
SIAD
syndrome of inappropriate antidiuretics
sNa
serum sodium