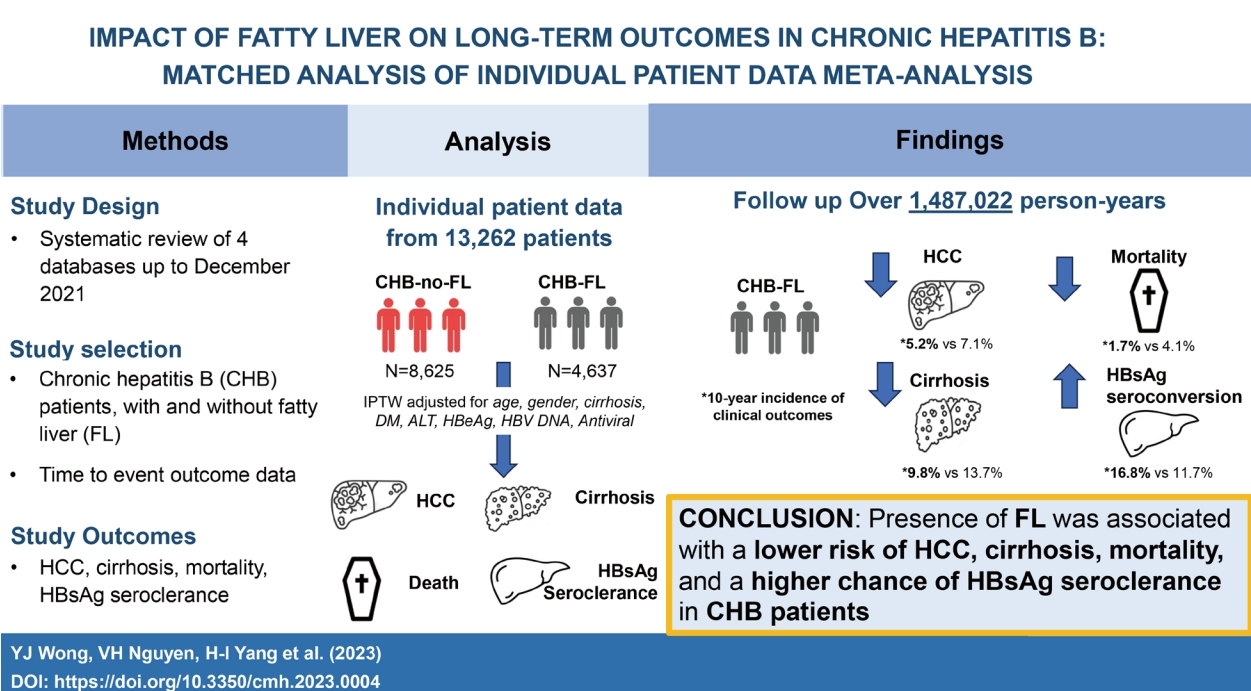
Impact of fatty liver on long-term outcomes in chronic hepatitis B: a systematic review and matched analysis of individual patient data meta-analysis
Article information
Abstract
Background/Aims
Chronic hepatitis B (CHB) and fatty liver (FL) often co-exist, but natural history data of this dual condition (CHB-FL) are sparse. Via a systematic review, conventional meta-analysis (MA) and individual patient-level data MA (IPDMA), we compared liver-related outcomes and mortality between CHB-FL and CHB-no FL patients.
Methods
We searched 4 databases from inception to December 2021 and pooled study-level estimates using a random-effects model for conventional MA. For IPDMA, we evaluated outcomes after balancing the two study groups with inverse probability treatment weighting (IPTW) on age, sex, cirrhosis, diabetes, ALT, HBeAg, HBV DNA, and antiviral treatment.
Results
We screened 2,157 articles and included 19 eligible studies (17,955 patients: 11,908 CHB-no FL; 6,047 CHB-FL) in conventional MA, which found severe heterogeneity (I2=88–95%) and no significant differences in HCC, cirrhosis, mortality, or HBsAg seroclearance incidence (P=0.27–0.93). IPDMA included 13,262 patients: 8,625 CHB-no FL and 4,637 CHB-FL patients who differed in several characteristics. The IPTW cohort included 6,955 CHB-no FL and 3,346 CHB-FL well-matched patients. CHB-FL patients (vs. CHB-no FL) had significantly lower HCC, cirrhosis, mortality and higher HBsAg seroclearance incidence (all P≤0.002), with consistent results in subgroups. CHB-FL diagnosed by liver biopsy had a higher 10-year cumulative HCC incidence than CHB-FL diagnosed with non-invasive methods (63.6% vs. 4.3%, P<0.0001).
Conclusions
IPDMA data with well-matched CHB patient groups showed that FL (vs. no FL) was associated with significantly lower HCC, cirrhosis, and mortality risk and higher HBsAg seroclearance probability.
Graphical Abstract
INTRODUCTION
Both chronic hepatitis B (CHB) and non-alcoholic fatty liver disease are leading causes of cirrhosis, hepatocellular carcinoma (HCC), and mortality worldwide [1,2]. However, while fatty liver (FL) co-exists in about 30% of patients with CHB [3,4], data regarding its impact on the natural history of CHB remain sparse and conflicting.
Higher risk of HCC has been reported among patients with CHB-FL as compared to patients with CHB without FL (CHB-no FL), especially in studies based on liver biopsy cohorts [5-11]. On the other hand, several large studies with long-term follow-up have reported a lower risk of HCC among CHB-FL patients compared to CHB-no FL patients [12,13]. Similar contradictory findings have also been observed in risk of cirrhosis [14], hepatic decompensation [8,15,16], mortality [7-9,13-19] as well as in the incidence of HBsAg seroclearance [20-26]. These findings are intriguing as one would expect the co-existence of two chronic liver diseases to result in worse outcomes, yet there are laboratory and clinical data suggesting the potential protective effects of FL in CHB patients [27-29]. We postulate the inconsistent data regarding the long-term outcomes of CHB-FL patients from prior studies to be due suboptimal study design (cross-sectional, case control vs. cohort studies), to patient selection bias from single-center or single-country experience, inadequate adjustment for confounders, inadequate follow-up and/or sample size to evaluate relatively rare events, especially HBsAg seroclearance [5,9,10,14,15].
We performed a systematic review, conventional metaanalysis (MA) and an individual patient-level data meta-analysis (IPDMA). In addition, we used an inverse probability treatment weighted (IPTW) cohort to compare various clinical outcomes between CHB-FL and CHB-no-FL in IPDMA [30]. Specifically, we aimed to compare the incidence of HCC, cirrhosis, mortality, and HBsAg seroclearance between CHB-FL and CHB-no FL to determine how presence of FL influences these outcomes.
MATERIALS AND METHODS
Search strategy and study inclusion criteria
We performed our systematic review and MA according to the Preferred Reporting Items for Systemic Reviews and Meta-analysis (PRISMA) guidelines (Supplementary Table 1) [31]. We searched PubMed, EMBASE, Web of Science and the Cochrane Library databases from inception to December 1, 2021, using a search strategy designed in collaboration with a medical librarian (CW). The details of our search strategy are provided in Supplementary Table 2. Briefly, our search strategy was based on a combination of keywords based on “fatty liver”, “steatosis”, “hepatitis B”, “hepatocellular carcinoma”, “cirrhosis”, “fibrosis”, “mortality”, and “HBsAg seroclearance”.
We included cohort studies that (1) included patients with CHB-FL and CHB-no FL aged 18 years or older, with FL diagnosed by either imaging (ultrasound, computed tomography scan or magnetic resonance imaging/spectroscopy), liver biopsy or central attenuation parameter (CAP) score with transient elastography and (2) provided time-to-event data on any of the clinical outcomes of interest (HCC, cirrhosis, mortality and/or HBsAg seroclearance). To estimate incidence and relative risk, time-to-event data are required; therefore, we excluded case-control studies and other cross-sectional study design. To avoid patient selection bias, we excluded randomized controlled trials. To avoid major confounding factors, we also excluded studies that did not exclude other liver diseases such as viral hepatitis C or excessive alcohol consumption. Additionally, we excluded studies that were review articles, editorials, case reports or guidelines, conference abstracts older than 2 years, animal or paediatric studies. If there were overlapping data from multiple studies from the same cohort, we included data from the largest, most comprehensive, and/or most updated study.
Two authors independently performed the initial screening of titles and abstracts identified in the primary search for eligibility, followed by full-text review. Discrepancies were resolved by consensus and/or with the third author as needed.
We also searched the bibliographies of relevant studies for potential additional studies. Authors of eligible studies were contacted to obtain additional aggregated data for conventional MA or de-identified individual patient-level data for inclusion in IPDMA if available.
Data collection and study quality assessment
Two authors also independently extracted the data of eligible studies using a standardized case report form developed for this study [32] and performed study quality assessment using a scale developed for this study that was based on the Newcastle-Ottawa Scale for cohort study (Supplementary Table 3) [33]. Studies with a score 7–9 were considered to be of high quality, 4–6 fair quality, and <4 poor quality. Discrepancies during data collection and study quality assessment were resolved by consensus and with a third author as needed.
We extracted data on baseline patient characteristics (age, sex, cirrhosis and diabetes mellitus, alanine aminotransferase [ALT], HBeAg, HBV DNA, and antiviral treatment status), study characteristics (publication date, study location, primary author, sample size, and study design), follow-up duration (person-years), and relevant clinical outcomes (HCC, cirrhosis, mortality and HBsAg seroclerance). If not reported by the study, we estimated the annual rate of the outcome of interest by dividing the number of patients with the outcome by the product of mean follow-up duration in years times the total number of patients and the person-years of follow-up by dividing the number of patients who developed the event by the annual incidence rate of said event.
Statistical analysis
The primary study outcomes were the comparative incidence of HCC, cirrhosis, mortality, and HBsAg seroclearance between CHB-FL and CHB-no FL patients.
First, we performed a conventional MA using aggregated study-level data to estimate and compare pooled incidence (per 1,000 person-years) of HCC, cirrhosis, mortality and HBsAg seroclearance between CHB-FL and CHB-no FL patients using a random-effects model. Pre-specified subgroup analyses based on diagnostic criteria of FL (biopsy vs. imaging vs. transient elastography), location of study (Asia vs. non-Asia), sample size, and treatment status were performed. We used the Cochran Q-statistic and I2 statistic to assess study heterogeneity with P<0.05 for Q-statistic and I2≥50% considered significant. The Egger’s test and funnel plot were used to assess for publication bias. All statistical analyses for conventional MA were performed using the meta packages in R statistical software (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria).
Second, we performed IPDMA. We contacted corresponding authors of studies included in conventional MA to provide individual patient data for IPDMA through email. Missing data were not analysed in IPDMA. We described patient baseline characteristics as median with interquartile range (IQR) or mean (±standard deviation [SD]) for continuous variables or as number and percentage for categorical variables. We compared patient baseline characteristics using the Student t-test or Wilcoxon signedrank test for continuous variables depending on their distribution and the Chi-squared test for categorical variables. Next, we balanced the CHB-FL and the CHB-no FL groups using IPTW method on age, sex, baseline cirrhosis, diabetes mellitus, ALT, HBeAg, log HBV DNA, and antiviral treatment status. We compared the incidence of HCC, cirrhosis, mortality, and HBsAg seroclearance in the IPTW-cohort (consist of both CHB-FL and CHB-no FL patients) using the Kaplan–Meier method and the log-rank test. Finally, we used Cox proportional hazard regression to estimate the hazard ratio (HR) relating FL to each of the study outcomes. All statistical analyses for IPDMA were performed using STATA version 16.0 (StataCorp LLC, College Station, TX, USA).
RESULTS
Conventional meta-analysis of aggregated study-level data
Search results and study characteristics
From the initial title and abstract review of the 2,157 records identified using our search strategy, 90 potentially eligible studies were retrieved for full-text assessment (Fig. 1). Our manual search of bibliographies of relevant articles provided eight additional potential studies [11,13,16,19,20-22,26]. In total, 19 studies involving 17,955 patients (6,047 CHB-FL, 11,908 CHB-no FL) met our inclusion criteria were included in the conventional MA [5-16,18,20-25]. A total 11 studies (14,014 patients) provided outcome data for HCC, 4 studies for cirrhosis (7,201 patients), 5 studies for mortality (9,266 patients), and 7 studies for HBsAg seroclearance (9,462 patients). The majority of the studies (73.7%, 14/19) were of high quality with the remaining of fair quality (Supplementary Table 3). There was no significant publication bias (Egger’s test P=0.85, Supplementary Fig. 1).
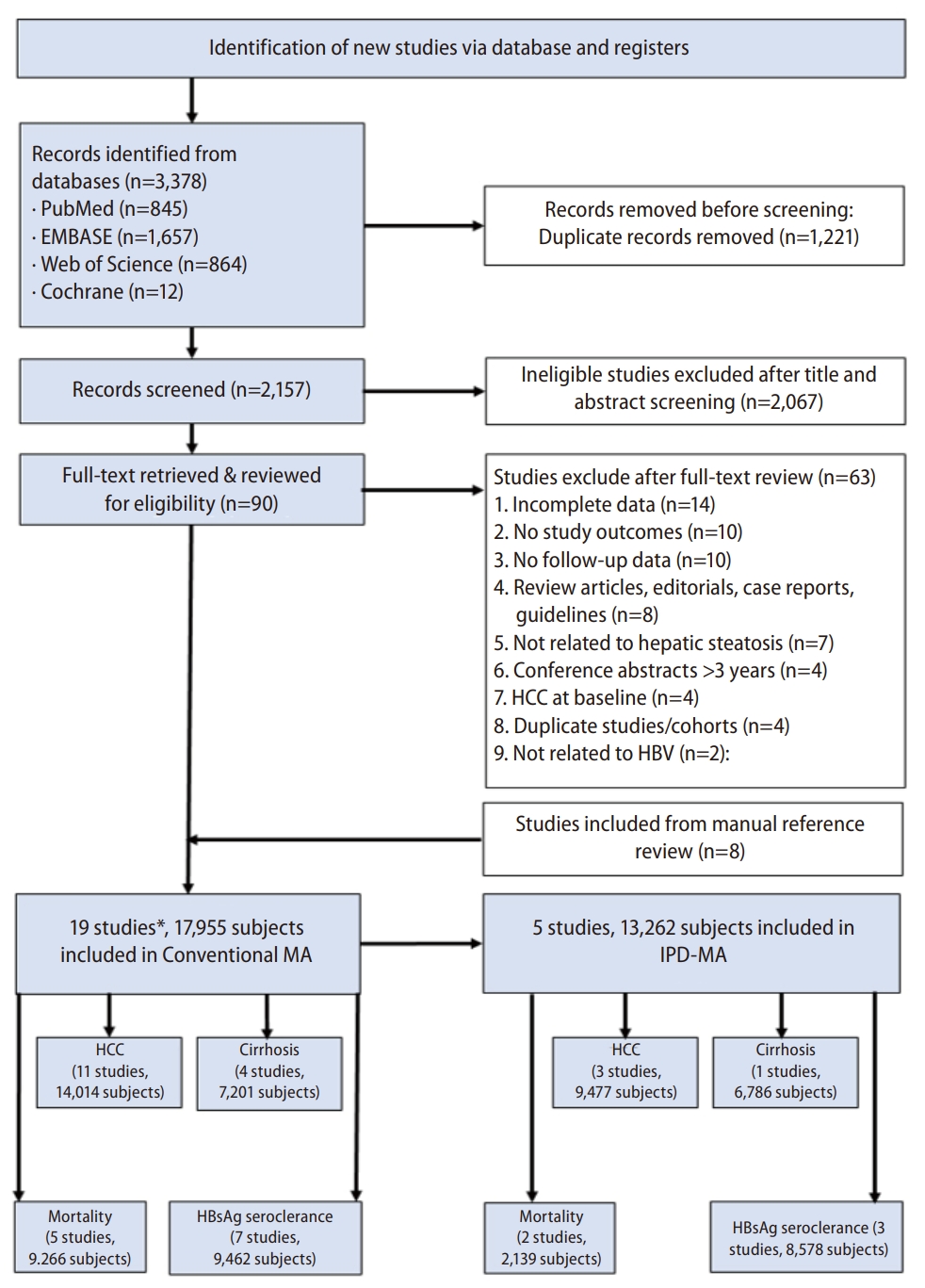
PRISMA flow diagram. HBV, hepatitis B virus, HCC, hepatocellular carcinoma, MA, meta-analysis, IPD-MA, individual patient data meta-analysis; HBsAg, hepatitis B surface antigen. *Some studies may have more than 1 study outcomes.
Six studies were prospective cohort studies and 13 were retrospective cohort studies (Supplementary Table 4). Sixteen were from Asia with 1 study each from North American, Europe, and Australia. All studies were published as full manuscripts except for one [11]. Majority of studies were single-center studies (89.5%, 17/19). The mean age of patients ranged between 38 and 60 years. The prevalence of FL varied between about 25% and 60% except for two studies that had prevalence ≤10% [14,18]. FL was most commonly diagnosed using ultrasound (3,031 participants, 6 studies) followed by transient elastography (2,119 participants, 6 studies) and liver biopsy (897 participants, 8 studies) (Supplementary Table 4).
Analysis of clinical outcomes
Overall incidence
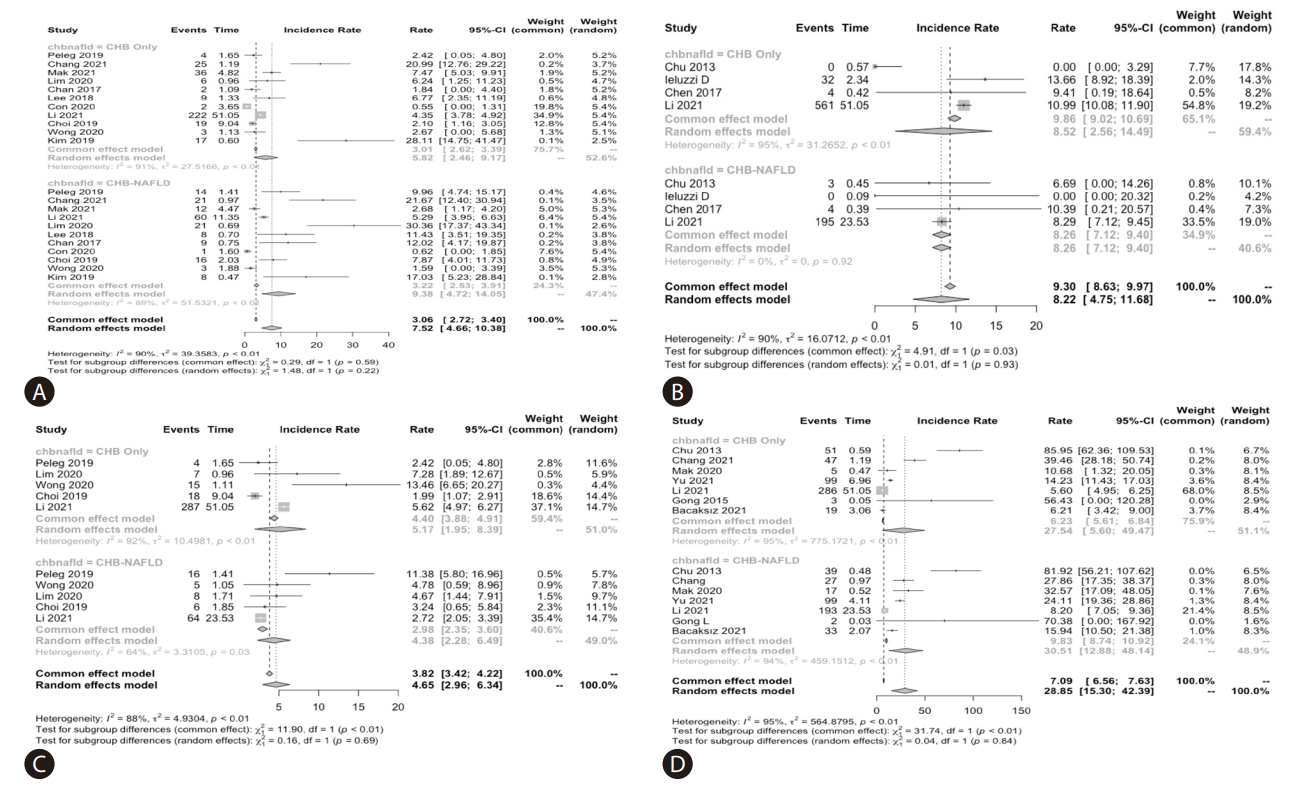
The pooled annual incidence of HCC, liver cirrhosis, mortality and HBsAg seroclearance of the total cohorts among the included studies were 7.6 per 1,000 person-years (95% confidence interval [CI]: 3.7–11.4; I2=93.1%; 11 studies, 14,014 participants), 9.3 per 1,000 person-years (95% CI: 4.1–14.6; I2=87.4%; 4 studies, 7,201 participants), 5.1 per 1,000 personyears (95% CI: 2.9–7.2; I2=90.7%; 5 studies, 9,266 participants), and 30.3 per 1,000 person-years (95% CI: 9.3–51.2; I2=99.0%; 7 studies, 9,462 participants).
The pooled incidence of all the above clinical outcomes were similar between CHB-FL and CHB-no FL patients based on aggregated data meta-analysis (P=0.27–0.93) (Table 1, Fig. 2). The heterogeneity was substantial in all analyses (I2=88–95%, P<0.01) (Fig. 2).
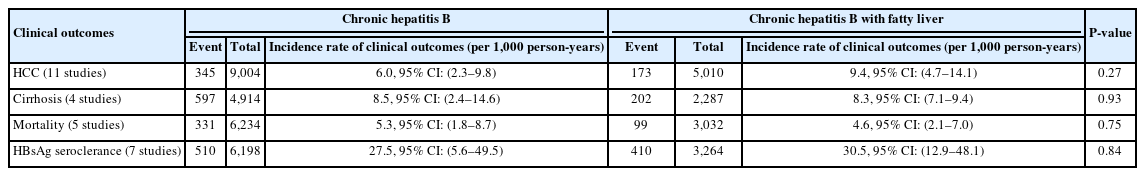
Conventional aggregated study-level data meta-analysis: Pooled incidence of clinical outcomes in chronic hepatitis B patients with or without concomitant fatty liver: (A) hepatocellular carcinoma, (B) cirrhosis, (C) Mortality, and (D) HBsAg seroclearance
Incidence in subgroups
There were no significant differences in the HCC incidence between CHB-FL and CHB-no FL patients in subgroup analysis by ethnicity, study sample size, or FL diagnosis methods, except for the subgroup with FL diagnosed by biopsy, where there was higher pooled HCC incidence (per 1,000 personsyears) for CHB-FL compared to CHB-no FL patients (12.6, 95% CI: 6.7–18.5, I2=63.5% vs. 2.9, 95% CI: 1.3–4.5, I2=39.2%, P=0.002, 5 studies, 2,493 participants) (Supplementary Table 5A). There was also significant difference between the treated, untreated, and mixed treated/untreated subgroups, but there was only one study providing stratified data for treated patients. Heterogeneity was severe with most analyses with I2 up to 98%.
There were only two studies providing data for subgroup analysis for cirrhosis outcome (Supplementary Table 5B). In subgroup analysis with more than one study in each subgroup, there was no significant difference among the sub groups by FL diagnosis method, ethnicity, study sample size or by antiviral treatment status, but heterogeneity was high in all analyses.
In most subgroup analyses for mortality outcome, data were available from only one study for most subgroup analyses (Supplementary Table 5C). In subgroup analyses with at least 2 or more studies in each study arm, there was no significant mortality difference between the study groups.
For HBsAg seroclearance outcome, there were 2 or more studies providing data for each of the study groups in most subgroup analyses (Supplementary Table 5D). The incidence rates of HBsAg seroclearance were similar between CHB-FL and CHB-no FL in all subgroup analysis, but there was severe heterogeneity in all analyses (I2 =74–99%).
Meta-analysis of individual patient-level data(IPDMA)
Total cohort
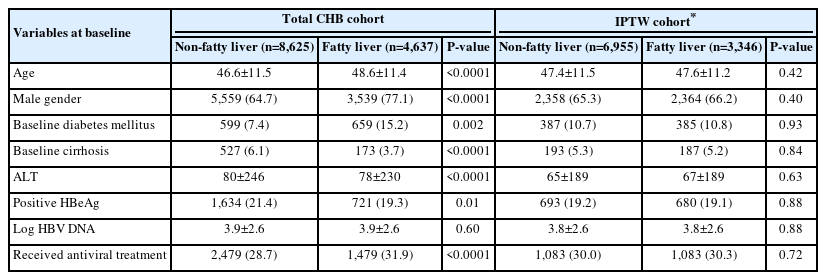
We included 13,262 patients (4,637 CHB-FL, 8,625 CHB-no FL) followed-up over 1,487,022 persons-years in our IPDMA (Fig. 1) [9,12,14,23,25]. Compared to CHB-no FL patients, CHB-FL patients were older (48.6±11.4 vs. 46.6±11.5 years, P<0.001), more likely male (77.1% vs. 64.7%, P<0.001), more likely to have diabetes mellitus, (15.2% vs. 7.4%, P=0.002), but less likely to have cirrhosis (3.7% vs. 6.1%, P<0.001) (Table 2). There were also statistically significant differences in their ALT levels, HBeAg status, and antiviral treatment status.
IPTW-weighting cohort
After IPTW weighting on age, sex, baseline cirrhosis, diabetes mellitus, ALT, HBeAg, HBV DNA, and antiviral treatment status, the IPTW cohort included a total of 10,301 patients (3,346 with CHB-FL and 6,955 with CHB-no FL) who were similar in their distribution of age, sex, baseline diabetes mellitus, baseline cirrhosis, ALT, HBeAg status, HBV DNA viral load, and antiviral treatment status (Table 2, Supplementary Fig. 2).
Overall incidence
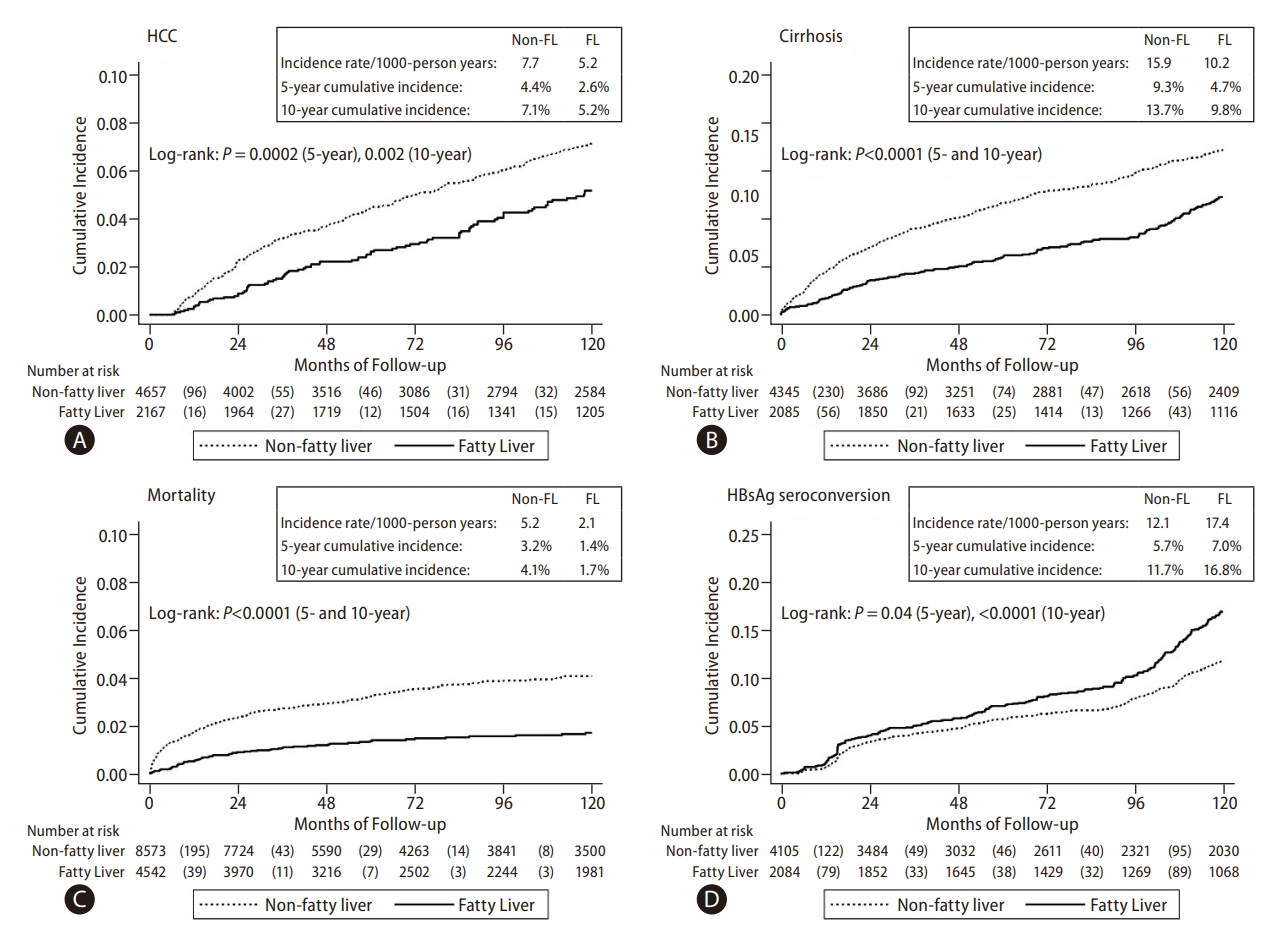
Compared to the CHB-no FL group, the CHB-FL had significantly lower incidence of HCC (P=0.002), cirrhosis (P<0.0001), and mortality (P<0.0001) while having significantly higher HBsAg seroclearance rate (P<0.0001) (Fig. 3). Incidence per 1,000 person-years in CHB-FL and CHB-no FL, respectively, were 5.2 and 7.7 for HCC, 10.2 and 15.9 for cirrhosis, 2.1 and 5.2 for mortality, and 17.4 and 12.1 for HBsAg seroclearance.
Incidence in subgroups
In subgroup analysis by age (Supplementary Fig. 3A, B) showed significantly lower HCC, cirrhosis, and mortality incidence and higher HBsAg seroclerance incidence in the CHB-FL group among patients older 45 years (P=0.005 for HCC, <0.0001 for all others). For patients aged 45 years or younger, the direction of difference was similar for all 4 outcomes but was statistically significant only for HBsAg seroclearance outcome (P=0.04). The 5-year and 10-year cumulative incidence for subgroup analyses were provided in Table 3.
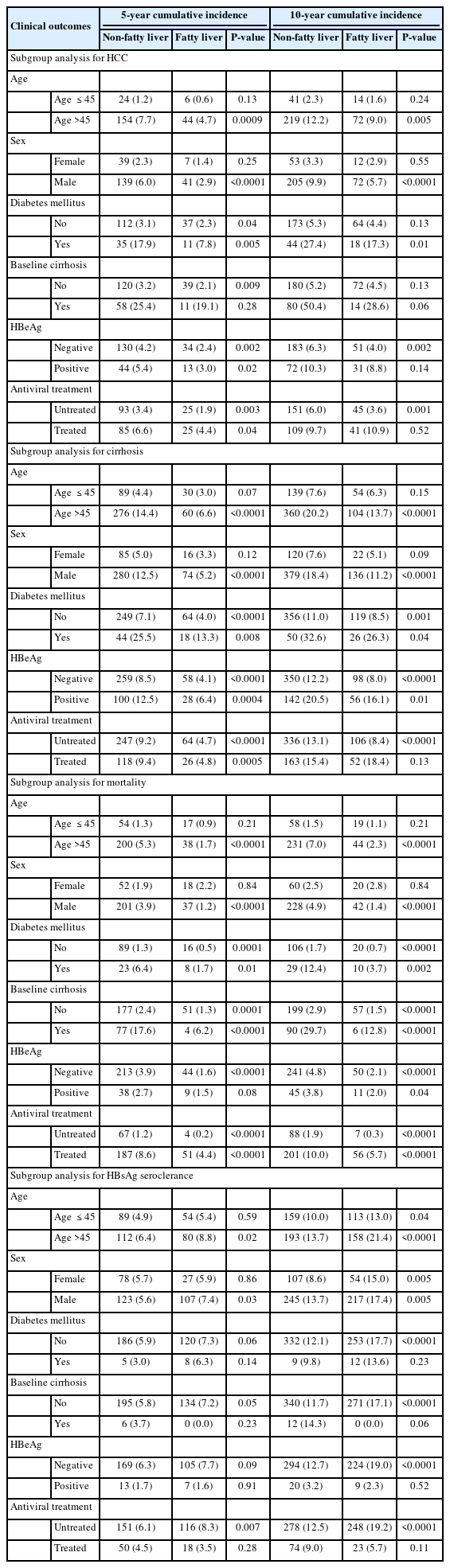
Individual patient-level data meta-analysis: Subgroup analysis for hepatocellular carcinoma, cirrhosis, mortality and HBsAg seroclerance in CHB patients with and without concomitant FLD
In subgroup analysis by sex (Supplementary Fig. 3C, D), HB sAg seroclearance incidence was significantly higher in CHB-FL group for both males (P=0.005) and females (P=0.005). While incidence of HCC, cirrhosis and mortality were significantly lower in CHB-FL for males (P<0.0001) there were no significant difference in the incidence of HCC, cirrhosis and mortality between CHB-FL and CHB-no FL in females.
In subgroup analysis by treatment status (Supplementary Fig. 3E, F), we found consistently lower HCC, cirrhosis, and mortality rates in CHB-FL (vs. CHB-no FL) among untreated patients (P<0.0001 for all), but not among their treated counterparts. Among treated patients, the 5-year cumulative incidence for CHB-FL vs. CHB-no FL groups of 4.4 vs. 6.6% for HCC (P=0.04), 4.8 vs. 9.4% for cirrhosis (P=0.0005), 4.4 vs. 8.6% for mortality (P<0.0001), and 3.5 vs. 4.5% for HBsAg seroclearance (P=0.28) (Table 3). Findings at 10-year follow-up were less consistent.
Findings were consistent for both diabetic and non-diabetic patients for most outcomes (Supplementary Fig. 3G, H, Table 3). Among HBeAg-positive patients, the CHB-FL group had a lower 5-year cumulative incidence for HCC (3.0 vs. 5.4%, P=0.02), cirrhosis (6.4 vs. 12.5%, P=0.0004), and mortality (1.5 vs. 2.7%, P=0.08). However, there was no significant difference in HBsAg seroclearance among HBeAg-positive patients with and without FL (5-year cumulative incidence 1.6 vs. 1.7%, P=0.91) (Table 3 and Supplementary Fig. 3I).
Subgroup analysis based on baseline cirrhosis status showed a consistently lower incidence for HCC and mortality and a higher HBsAg seroclearance in CHB-FL patients (vs. CHB-no FL patients) (Supplementary Fig. 3J, K, Table 3).
Notably, in subgroup analysis of HCC by FL diagnosis method, we found a significantly higher HCC incidence among CHB-FL patients diagnosed by liver biopsy as compared to CHB-FL patients diagnosed by noninvasive methods (10-year cumulative incidence: 63.6% vs. 4.3%, P<0.0001; Supplementary Fig. 3L). While the odds of patients with baseline cirrhosis were comparable between the CHB-FL and CHB-no-FL groups among non-biopsy cohorts (odds ration [OR] 1.19, 95% CI: 0.72–1.98, I2=59%), the biopsy cohort had a higher odd of having patients with cirrhosis at baseline in the CHB-FL group than in the CHB-no-FL group (OR 1.68, 95% CI: 1.27–2.23, I2=2%) (Supplementary Fig. 4).
Factors associated with HCC, cirrhosis, mortality, and HBsAg seroclearance
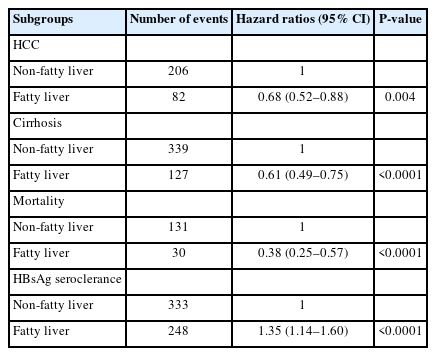
On Cox regression analysis (Table 4), CHB-FL was associated with significantly lower incidence of HCC (HR=0.68, 95% CI 0.52–0.88, P=0.004), lower incidence of cirrhosis (HR=0.61, 95% CI 0.49–0.75, P<0.001), lower incidence of mortality (HR=0.38, 95% CI 0.25–0.57, P<0.001), yet a higher incidence of HBsAg seroclearance (HR=1.35, 95%CI 1.14–1.60, P<0.0001) when compared to CHB-no FL.
DISCUSSION
In the current study, our conventional MA of 19 eligible studies involving 17,955 patients found no significant difference in the risk of HCC, cirrhosis, mortality, and HBsAg seroclearance between CHB-no FL and CHB-FL patients; however, these results were limited with severe heterogeneity in all analyses. On the other hand, our IPDMA using the IPTW cohort of 10,301 patients were all in favour of the CHB-FL population in all evaluated outcomes as compared to their CHB-no FL counterparts. These findings also held on several subgroup analyses. Taken together, our findings strongly suggest a protective effect of FL in patients with CHB.
A notable finding from our study is the markedly higher HCC incidence in studies based on biopsy cohorts compared to cohorts with FL diagnosed by non-invasive method. We also found that CHB-FL patients among biopsy cohorts were about 70% more likely to have cirrhosis at baseline than the CHB-no FL patients, while there was no significant difference in the distribution of cirrhosis between the two groups among non-biopsy cohorts, suggesting a potential selection bias among studies based on biopsy. Furthermore, in our conventional MA, the heterogeneity was lowest in the subgroup analyses comparing liver biopsy vs. non-invasive studies (I2=39.2% vs. 63.5%). These findings suggest that the diagnostic method of FL is likely the major source of heterogeneity. Given the invasive nature of liver biopsy, liver biopsies would not be used routinely in real-world practice, so that the biopsy cohort is likely subjected to selection and indication bias, and therefore may not be representative of all CHB patients. On the other hand, we acknowledge that non-invasive diagnosis of FL may be less accurate than liver biopsy. Nevertheless, an invasive method such as liver biopsy is neither ethical nor practical for large-scale epidemiological and clinical studies.
CHB-FL patients were more likely male, older and more likely have diabetes mellitus, all of which are known risks factors for HCC, yet these confounding factors were not adequately adjusted in prior studies [5,8]. In our IPDMA, we balanced the FL and non-FL groups using IPTW matching on background risks as well as antiviral therapy, thus making our results more robust and reliable. Additionally, we only included cohort studies with follow-up data so we could accurately estimate all time-to-event outcomes.
We found that the CHB-FL group had a significantly lower risk of mortality in all subgroups, except for female and those younger than 45 years old. This finding is line with prior studies of NAFLD, which found no significant or lower mortality in patients with NAFLD unless they have advanced fibrosis [34,35]. While it may seem paradoxical as one would expect a higher incidence of liver-related death in CHB-FL patients because both CHB and NAFLD were independent driver for liver fibrosis, the mortality in NAFLD was driven by non-liver-related death in most of the studies [8,9,15] except one [7].
Another key finding of our study was that the CHB-FL group had a higher incidence of HBsAg seroclearance than the CHB-no FL group. Our finding is in line with prior study where, the presence of obesity, a common occurrence in FL, was an independent predictor for HBsAg seroclearance [36]. Meanwhile, the overall HBsAg seroclearance rate in our CHB-no FL cohort were higher than the rate observed in the Alaska cohort (14.0 vs. 5.6 per 1,000 person-years) [37]. This finding is intriguing as Caucasians generally had a higher rate of HBsAg seroconversion. We hypothesized that this may be due to the differences in median age (47.4 vs. 19.9 years) and a predominantly East Asian ethnicity of our IPDMA cohort, as older age has been reported as an independent predictor for HBsAg seroclearance [38].
Our study yields a different conclusion from those by a recent meta-analysis by Mao and colleague [39] which reported a higher risk of HCC in CHB-FL patients, likely due to differences in study methodology and inclusion criteria. First, our study exclusively included study with time-to-event data, which is the appropriate method to summarize time-to-event data such as incidence data for HCC development, HBsAg Seroclearance, and death [37]; whilst Mao et al. [39] also included studies that reported outcomes as proportions rather than timeto-event data. Additionally, Mao et al. [39] combined proportion data with time-to-event data and estimated their pooled effect by pooling together odds ratio, relative risk and hazard ratio, which are measurements that are not compatible, and should not have been pooled together [40], thus limiting their provide time-to-event data [41-45]. Second, Mao et al. [39] included a recent administrative database study with 48,335 CHB patients reported a higher risk of HCC and death in CHB-FL group, which is contrasting to our findings [19]. However, this study included both hepatitis B and C patients, and subgroup analysis of CHB patients did not provide time-to-event data to be included in our study and did not provide details on which factors were being adjusted for [19]. There were also no laboratory data to account for differences in comparative groups in regards to established risk factors for HCC such as HBV DNA, baseline HBeAg status as well as serum ALT [34]. Moreover, administrative database is prone to miscoding. We addressed these limitations in our study with the use of IPDMA based on medical chart review of individual patients and careful adjustment of relevant confounders (including HBV DNA, HBeAg status and ALT) for HCC using IPTW analysis. Third, our conventional MA analysis included additional relevant studies reporting HBsAg seroclerance that were not included in the study by Mao et al. [39], one of which reported higher HBsAg Seroclearance rate and contributed data to our IPDMA.
NAFLD-associated metabolic stress can activate HBV-suppressed innate and adaptive immunity to eliminate HBV virus, thus delaying disease progression in CHB patients through several mechanisms. Saturated fatty acids have been shown to upregulate Toll-like-receptor 4 and activate myeloid differentiation factor 88-mediated pathway to inhibit the HBV replication [46]. An increased in FAS receptor on the membrane of hepatocytes with FL may lead to steatosisinduced apoptosis of hepatocytes and a higher chance of HBsAg seroclearance in CHB-FL patients [28]. Fat infiltration within the cytoplasm of infected hepatocytes may reduce the expression of HBsAg within cytoplasm the infected hepatocytes [28]. A lower HBV DNA load among CHB-FL patients compared to CHB-no FL patients has been demonstrated in both randomized trial and meta-analysis [29]. Given that reduction of HBV DNA is expected to be a pre-requisite for HBsAg seroclearance, these findings support a high incidence of HBsAg seroclearance in CHB-FL. The beneficial effect of FL on HBsAg seroclearance may explained the inverse relationship between FL and the reduction in cirrhosis and HCC. Ideally, these findings should be validated in large scale prospective studies, but this would be impractical considering the low incidence of HBsAg seroclearance among unselected CHB patients. Future studies examining the impact of FL on the trend of quantitative HBsAg as a surrogate for HBsAg seroclearance may help to clarify this finding [47].
The limitation of our IPDMA is that, despite its size, the number of patients for rarer outcomes such as HBsAg seroclearance among young or HBeAg seropositive patients remained limited. As most of our IPDMA data were from Asian studies, our findings may not be generalized to non-Asian CHB patients. We acknowledge there are limitations on the non-invasive diagnosis of FL which were subjected to performance and operator bias and the cut-off for the diagnosis of FL may have varied across different studies.
In conclusion, data from well-matched CHB patients show that the presence of FL was independently associated with a lower risk of HCC, cirrhosis and mortality, and a higher chance of HBsAg seroclearance. By adjusting for potential confounders, our IPDMA provided a more robust and in-depth analysis than the conventional MA to elucidate the clinical impact of FL in CHB patients. Further studies are needed to elucidate the biological interaction between CHB and FL which can inform future therapeutic development and strategies for both diseases.
Notes
Authors’ contribution
Guarantor of article: Mindie H. Nguyen. Specific author contributions: Study concept: Yu Jun Wong and Mindie H. Nguyen. Study design: Yu Jun Wong, Vy Nguyen, Jie Li, Michael H. Le, Mindie H. Nguyen. Data collection: all authors. Data analysis: Vy Nguyen, Michael H. Le, Yu Jun Wong, Mindie H. Nguyen. Drafting of the article: Yu Jun Wong, Vy Nguyen, Michael H. Le, Mindie H. Nguyen. Data interpretation, review, and revision of the manuscript: all authors. Study supervision: Mindie H. Nguyen.
Conflicts of Interest
WYJ: Research/grant: Nurturing Clinician Scientist Scheme, Medicine Academic Clinical Program, SingHealth, Speaker’s fee: Gilead & AbbVie; RK: Research/grant: SingHealth foundation grant, Consulting/advisory board: Gilead Sciences, Speaker bureau: Gilead Sciences, AbbVie; VW: Consultancy: AbbVie, Boehringer Ingelheim, Echosens, Intercept, Inventiva, Novo Nordisk, Pfizer, TARGET PharmaSolutions, Lectures: Abbott, AbbVie, Gilead Sciences, Novo Nordisk, Research grants: Gilead Sciences, Stock: Co-founder of Illuminatio Medical Technology Limited; GW: Research/grant: Gilead Sciences, Consulting/Advisory board: Gilead Sciences, Janssen, Speaker’s bureau: Abbott, AbbVie, Bristol-Myers Squibb, Echosens & Furui, Gilead, Janssen, Roche; MHN: Research support: Pfizer, Enanta, CurveBio, Gilead, Exact Sciences, Vir Biotech, Helio Health, National Cancer Institute, Glycotest, B.K. Kee Foundation; Consulting and/or Advisory Board: Intercept, Exact Science, Gilead, GSK, Eli Lilly, Laboratory of Advanced Medicine, Janssen
Acknowledgements
We would like to thank Professor Seto Wai-Kay, Dr Mak Lungyi and Professor Yuen Man Fung for sharing the individual patient data for this study.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
PRISMA checklist for systematic reviews and meta-analyses
Search strategy
Quality assessment of observational studies using Newcastle–Ottawa Scale
Summary of included studies on chronic hepatitis B with or without fatty liver
Aggregated study-level data meta-analysis: Subgroup analysis for (A) Hepatocellular carcinoma, (B) cirrhosis, (C) mortality, and (D) HBsAg seroclerance in CHB patients with and without concomitant FLD (A) Hepatocellular carcinoma
Funnel plot of conventional meta-analysis of 19 studies evaluating the risk of hepatocellular carcinoma between chronic hepatitis B patients with and without fatty liver.
Test of positivity assumption for the IPTW model. IPTW, inverse probability treatment weighting.
Individual patient-level data meta-analysis: Subgroup analysis of cumulative incidence of clinical outcomes in the total cohort of patients with chronic hepatitis B with and without fatty liver based on: Age (A & B), Sex (C & D), Treatment status (E & F), Presence of Diabetes Mellitus (G & H), HBeAg seropositivity (I), Baseline liver cirrhosis (J & K) and Diagnosis method of fatty liver (L).
Factors associated with baseline cirrhosis among chronic hepatitis B patients with and without fatty liver, stratified by diagnosis method of fatty liver. CHB, chronic hepatitis B; FL, fatty liver.
Abbreviations
NAFLD
non-alcoholic fatty liver disease
HBV
hepatitis B virus
CHB
chronic hepatitis B
FL
fatty liver
HCC
hepatocellular carcinoma
References
Article information Continued
Notes
Study Highlights
• What is already known in this topic?
The impact of fatty liver (FL) on the long-term outcomes of chronic hepatitis B (CHB) (cirrhosis, HCC, mortality and HBsAg seroclearance) in CHB remained controversial.
• What this study adds?
Pooled data from individual patient data meta-analysis (IPDMA) showed CHB patients with FL had a lower incidence of HCC, cirrhosis, and mortality risk, and higher chance of HBsAg seroclearance compared to CHB patients without FL (all P≤0.002). CHB-FL diagnosed via liver biopsy have much higher 10-year cumulative HCC incidence compared to those without FL.
• How this study may affect research, practice or policy?
Further studies are needed to elucidate the mechanistic interaction between CHB and FL.