A decade of liver organoids: Advances in disease modeling
Article information
Abstract
Liver organoids are three-dimensional cellular tissue models in which cells interact to form unique structures in culture. During the past 10 years, liver organoids with various cellular compositions, structural features, and functional properties have been described. Methods to create these advanced human cell models range from simple tissue culture techniques to complex bioengineering approaches. Liver organoid culture platforms have been used in various research fields, from modeling liver diseases to regenerative therapy. This review discusses how liver organoids are used to model disease, including hereditary liver diseases, primary liver cancer, viral hepatitis, and nonalcoholic fatty liver disease. Specifically, we focus on studies that used either of two widely adopted approaches: differentiation from pluripotent stem cells or epithelial organoids cultured from patient tissues. These approaches have enabled the generation of advanced human liver models and, more importantly, the establishment of patient-tailored models for evaluating disease phenotypes and therapeutic responses at the individual level.
INTRODUCTION
The liver is a complex organ that is vital for regulating metabolism, energy storage, and detoxification in the human body [1]. The liver is the largest internal organ, and it mainly comprises hepatocytes that are central to the performance of most of its functions [2,3]. Other cell types in liver tissue include parenchymal cholangiocytes and nonparenchymal liver cells (NPCs): liver sinusoidal endothelial cells, hepatic stellate cells (HSCs), Kupffer cells (KCs), and other immune cell types. Each cell type plays different roles, and together, they maintain the structural integrity of liver tissue during homeostasis and make repairs when needed due to injury or disease [4,5]. These cells are spatially organized into lobules and form channels, including the sinusoid and bile canaliculi networks, that are essential for transporting nutrients into the liver and exporting metabolites and bile into the digestive system. In addition to the loss of hepatocytes, disruption of these liver structures and cellular interactions often occurs during drug-induced liver injury (DILI) and liver disease, resulting in the loss of liver function.
Chronic liver disease (CLD) remains a leading cause of death and morbidity worldwide [6]. CLD is the major contributor to liver injury, fibrosis development, and the eventual progression to cirrhosis and primary liver cancer (PLC). Historically, the main etiology for CLD is viral hepatitis. However, with the development of antiviral treatments and vaccinations, a pandemic of obesity, and increased alcohol consumption, the primary emerging CLD etiologies are nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), and DILI [6,7]. Due to the complex interplay among the cellular components of the liver in varying disease conditions, it has been challenging to construct a robust human model for understanding disease manifestations and treatment responses [8,9]. Most human liver disease models have focused on the ways in which diseases and chemical agents induce hepatocyte injury and cell death. However, apart from those cellular changes, remodeling of the hepatic extracellular matrix (ECM) also plays an integral role in disease progression. Fibrogenesis is a common pathophysiological feature in injured and diseased livers, and it involves extensive ECM remodeling by activated HSCs [10]. These cells secrete fibril-associated collagens and matrix metalloproteinases that gradually remodel the soft and perforative endothelial/epithelial basal lamina, making it rigid and obscure. To accurately reflect disease progression, it is essential for research models to capture such ECM changes as they are driven by interactions among various cell-types.
Scientists have long used three-dimensional (3D) culture methods to create human organ models that mimic in vivo niches and promote multidimensional cell–cell and cell–environment interactions [11,12]. In recent years, two cell culture platforms for generating such 3D cellular models have been increasingly adopted by laboratories across various research fields: pluripotent stem cell (PSC)-derived organoids and tissue-derived organoids. Since their discovery, PSCs have been an invaluable cell source for creating almost all cell types in the human body [13,14]. PSCs have a limitless self-renewing capacity and the ability to mimic embryogenesis and give rise to adult tissue cell types, and they are highly amenable to gene editing, so they are a preferred starting cell source for generating human organ models. One of the first 3D approaches to initiate differentiation into various germ layers is embryoid body formation, in which cells of all three germ layers begin to form [15]. It remains an initiation step in many differentiation protocols for PSC-derived organoids. To date, organoids resembling the brain [16], lung [17], kidney [18], stomach [19], intestines [20], pancreas [21], and liver [22,23] have been generated. The advent of human induced pluripotent stem cell (iPSC) reprogramming technology, which enables the generation of patient-specific PSC cells, has further accelerated the adoption and utility of the PSC-derived organoid platform [24].
The ability of isolated adult mammalian cells to reassemble themselves into functional tissues when embedded in a culture matrix was first demonstrated using a 3D culture of epithelial cells derived from mammary glands and lung alveoli [25,26]. Both studies highlighted the ability of basement membrane matrix secreted from Engelbreth-Holm-Swarm sarcoma (commonly known as Matrigel) to support 3D human cell culture and the marked improvements in functioning shown by the 3D cultured cells compared with their twodimensional (2D) counterparts. In a landmark study by the Hans Clevers group, a single adult crypt stem cell was shown to generate functional intestine crypt organoids in Matrigel culture [27]. Specifically, those authors showed that by supplementing the cultures with growth factors and small molecules that modulate essential signaling pathways supported by the tissue niche, adult stem cells marked by Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) could be cultured and propagated for an extended period. Clevers’s group and others quickly showed that by manipulating culture media to activate essential signaling pathways that support progenitor cells in other tissue types, the platform could be adopted to derive organoid cultures from other organs, including the liver [28-30]. Remarkably, the tissue-derived organoid platform is also highly suitable for deriving cancer cells from tumors of various indications. These 3D cancer cell cultures, commonly called tumoroids, have facilitated a breakthrough in deriving patient-specific cancer cells for colon, pancreatic, liver, ovarian, and nasopharyngeal cancer [31-35].
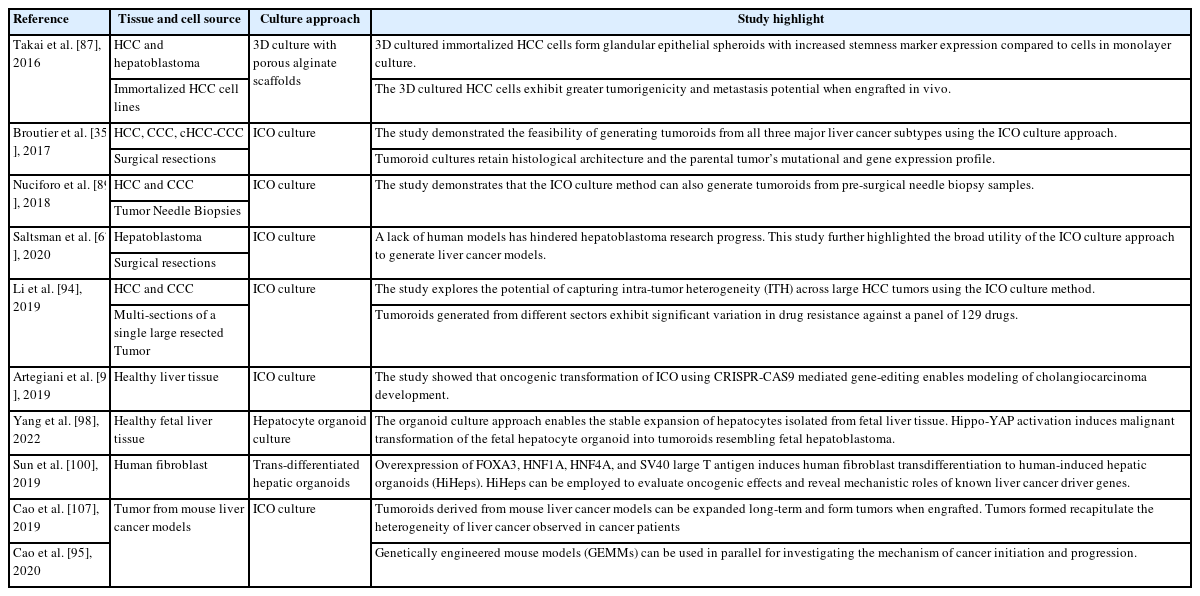
Human models, including immortalized hepatic cell lines, PSC-derived hepatocyte-like cells, and primary human hepatocytes (PHHs), have been widely used to study the pathogenesis of liver disease and to screen and evaluate treatment strategies [36,37]. Each model has its limitations and advantages. For instance, PHHs can preserve the genetic information and metabolic function of their in vivo counterparts. They are suitable for studying toxicity, metabolism, viral infection, and congenital disease. However, their adoption is significantly constrained by a lack of donors and limited proliferation capacity in vitro. 38 On the other hand, immortalized cell lines derived from hepatoblastoma (hepG2) or hepatocellular carcinoma (HCC) (Hep3B) provide a renewable cell source, but they lack multiple hepatocyte functions. Their cancerous features and genomic instability also remain a concern for many applications [39]. During the past decade, hepatic organoids have emerged as a new human in vitro option for modeling multiple liver diseases, including hepatitis, NAFLD, ALD, monogenic disorder, and primary liver cancer (PLC) (Tables 1–4). In general, liver organoids have significantly increased the feasibility of establishing patient-specific models. Additionally, they incorporate multiple liver cell types to model disease pathophysiology and enable the recapitulation of structural changes observed during tissue injury and disease progression. Liver organoids of different cellular compositions, sizes, structures, and functions can be derived from PSCs and human liver tissue (Fig. 1) [40,41]. In this review, we follow the consensus nomenclature of Marsee et al. [42],which reflects the cellular origins of tissues and the cellular composition and function of the organoid. Several reviews have provided in-depth discussions about how various liver organoids (Fig. 1) can be generated using different cell culture systems [40,41]. In this review, we focus on how PSC-derived and tissue-derived liver organoid systems are being used to advance the study of major liver diseases.
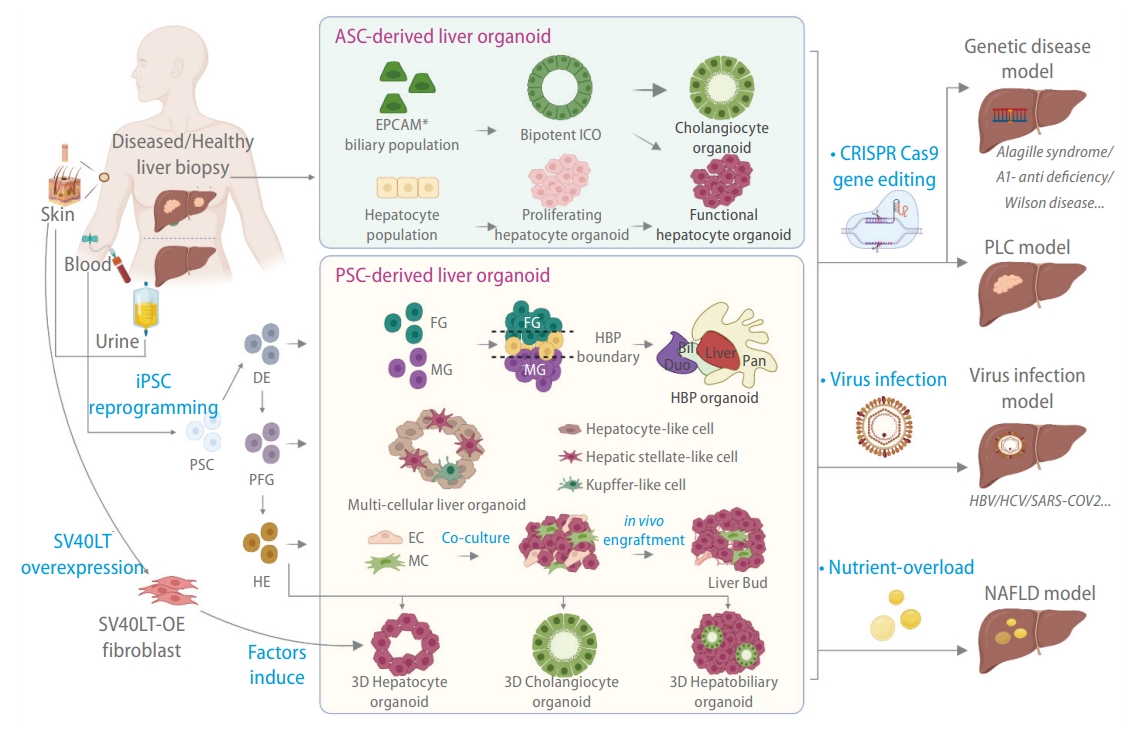
Methods for generating liver organoids for disease modeling. Liver organoids can be broadly classified into adult stem cell (ASC)-derived liver organoids and pluripotent stem cell (PSC)-derived liver organoids. ASC-derived liver organoids are generated by stem-like cells derived from tissue biopsies. Bipotent intrahepatic cholangiocyte organoids (ICOs) can be derived from EPCAM+ biliary cell populations isolated from diseased or healthy liver biopsies. Such cells tend to be refractory toward hepatocyte differentiation. The hepatocyte population from a liver biopsy can be dedifferentiated into proliferating hepatocyte organoids that can be stably propagated and subsequently induced to form functional hepatocytes. Patient-derived iPSCs can generate different organoid types via a stepwise differentiation strategy along the endoderm lineage (DE, definitive endoderm; PFG, posterior foregut; HE, hepatic endoderm). This includes multi-tissue hepato-biliary-pancreatic (HBP) organoids, multi-cellular organoids containing both liver parenchymal (hepatocytes) and non-parenchymal (hepatic stellate cells [HSCs] and Kupffer cells [KCs]) cell types, hepatobiliary organoids containing parenchymal hepatocytes and cholangiocytes, and single cell type hepatocyte or cholangiocyte organoids. HE can also be co-cultured with endothelial cells (ECs) and mesenchymal cells (MCs) to form a liver bud that generates vascularized tissue when engrafted. Fibroblast over-expressing (OE) SV40 large T antigen (SV40LT) can be directly transdifferentiated into hepatocyte organoids with a combination of hepatocyte-inducing factors. The organoids can be subsequently manipulated in culture to model various liver diseases.
HEREDITARY LIVER DISEASES
Monogenic liver diseases are caused by single-gene mutations. These diseases include conditions that directly induce injury in liver parenchymal cells and diseases in which the liver is intact and the phenotype develops in other parts of the body [43]. The prevalence of individual diseases caused by a single-gene mutation is low, with such diseases occurring in approximately 1% of newborns [43]. Current pharmacological interventions include enzyme replacement therapy and chelation therapy to enhance toxin excretion [44,45]. Unfortunately, those therapies are usually expensive and have poor cost-effectiveness. Gene therapy has emerged as a promising therapeutic option [46]. However, the application of such therapy is limited by the efficacy of the delivery system and the expression of the therapeutic gene. In addition, adverse immune responses and safety concerns about the delivery vectors pose significant difficulties to clinical translation. Liver transplantation remains the standard intervention for many hereditary liver diseases.
The successful derivation of organoids from diseased tissue provides a new resource for modeling and understanding hereditary disease and searching for novel therapeutic targets (Table 1). Organoids derived from diseased tissues recapitulate the genomic backgrounds of individual patients and retain the functional dysregulations of monogenetic disorders [28,47-49]. In addition, genome editing technology enables the precise insertion and correction of mutated sequences for further mechanistic study. Clevers’s group reported the first platform for isolating bipotent intrahepatic cholangiocyte organoids (ICOs) from healthy and diseased tissues [47]. Bipotent ICOs can be further differentiated in vitro into functional hepatocytes that secrete albumin, exhibit CYP3A4 activity, and give rise to new hepatocytes upon engraftment in mice. This platform thus makes it possible to model adult liver functions and mimic disease pathophysiology in vitro. Those authors first demonstrated that possibility by isolating diseased organoids from patients with a1-antitrypsin (A1AT) deficiency or Alagille syndrome (ALGS). A1AT deficiency is a genetic disorder caused by the defective production of the A1AT protein in hepatocytes [47]. The A1AT protein is secreted in the lungs to protect the lungs against damage caused by the neutrophil elastase. A1AT deficiency is characterized by an excessive accumulation of abnormal A1AT protein in the liver and reduced A1AT levels in the lung, resulting in the injury to the alveoli [49,50]. Differentiated organoids from A1AT patients displayed normal albumin secretion and low-density lipoprotein (LDL) uptake ability, and the cells acquired the hallmarks of A1AT-deficient patients [47,49]. The patient-derived organoids exhibited reduced A1AT secretion compared with the healthy control organoids, and aggregates of abnormal A1AT increased. These phenotypes are similar to the phenotypes observed in diseased tissue. ALGS is a common monogenic liver disease resulting from a loss-of-function mutation in the JAG1 gene; it affects various organs, particularly the heart, liver, eyes, and vertebrae. The JAG-NOTCH signaling pathway is highly conserved and critical in the development of multiple organs and determination of cellular fates. The clinical manifestation of ALGS in the liver includes bile duct malformation [51]. Patients can present with impaired biliary differentiation, duct paucity, or chronic cholestasis. Isolated patient bipotent organoids are indistinguishable from healthy organoids. However, upon differentiation, the patient organoids failed to upregulate cholangiocyte markers such as cytokeratin 19 and cytokeratin 7 [47]. Subsequently, the organoids lost their morphology and underwent apoptosis. In summary, that pioneering study offered a novel approach for deriving renewable, patient-specific, primary cell models. The rapid adoption of that technique by other research teams for similar genetic liver disease studies supports the robustness of the ICO platform. Gómez‐Mariano’s team isolated diseased ICOs from patients with heterozygous and homozygous mutations in the A1AT-encoding gene SERPINA1, and the organoids with corresponding genotypes exhibited different protein deficiency levels [49]. Those authors further demonstrated that the organoids could respond to external stimuli, such as oncostatin M, which is a well-known inducer of A1AT expression. Other teams have further extended the application of this technique to animal models, including genetically engineered mouse models (GEMMs) [51] and large animal models such as canines (Table 1) [52,53].
iPSC technology was a landmark breakthrough that enabled the efficient establishment of patient-specific cell models [54]. Models of genetic liver diseases can be established using iPSCs generated from patients with the disease or via gene editing of healthy iPSC cell lines. Liver organoid models of those diseases can subsequently be established from the iPSCs using various differentiation strategies (Fig. 1). PSC-derived organoids range from simple single-cell-type hepatocyte or cholangiocyte organoids with cystic structures [55-57] to more complex multicellular organoids such as parenchymal hepatobiliary organoids and multicellular liver organoids containing both parenchymal and nonparenchymal cell types [23,58,59]. The structural features present in the organoids allow researchers to recapitulate disease phenotypes in a dish. Harnessing the cystic structure formed by cholangiocyte-like cell (CLC) organoids, Sampazio et al. [56] were able to model the disruption of molecules and fluid transport that occur in cholangiopathy-inducing genetic diseases such as ALGS, polycystic liver disease, and cystic fibrosis (CF). CF is one of the most common autosomal-recessive genetic diseases in the Western population and is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene [60]. The most frequent CF mutation seen in patients is the deletion of phenylalanine 508 (ΔF508), which results in rapid endoplasmic reticulum degradation of misfolded CFTR. The loss of CFTR results in the disruption of chloride transport in cells. CLC organoids derived from the iPSCs of CF patients exhibited disrupted intracellular chloride transport and chloride transport into the organoid lumen. Given the close relationship between chloride transport and fluid secretion in cholangiocytes, those authors further demonstrated that chloride transport dysfunction also affected fluid transport into the organoid lumen. Notably, they demonstrated that the organoids could be used to validate the efficacy of the experimental drug VX809, which is currently in phase III clinical trials. Using a similar strategy, other researchers have reported iPSC-derived liver organoid models of ALGS [61], citrullinemia type 1 [62], and Wolman disease [58]. All those liver organoids exhibited the hallmarks of their respective genetic disease, and subsequent correction with gene-editing approaches reversed the phenotypes [61]. Although many of those proof-of-concept studies demonstrated the potential of generating liver organoids with patient-specific genotypes, how those organoids could advance the study of monogenic liver diseases or replacement therapy remains to be explored. For example, future studies could examine how the different cell types within the liver interact to modulate disease development and progression. In addition, many of the iPSC-derived organoids described herein face the inherent challenge that the hepatocytes generated are immature [63]. These PSC-derived cells are often referred to as hepatocyte-like cells (HLCs) because they closely resemble fetal liver hepatocytes and express markers such as alpha-fetoprotein (AFP) that are absent from adult liver hepatocytes. Of concern, these immature cells might also lack adult hepatocyte functions, such as selected drug-induced cytochrome p450 activity [64]. Significant progress has been achieved in identifying the molecular pathways necessary to induce maturity in fetal hepatocytes, and subsequent optimized culture conditions have enabled considerable progress in PSC-derived HLCs [65]. Therefore, culture conditions for liver organoid models could similarly be optimized in the future to enhance the functional maturity of the cells.
PRIMARY LIVER CANCER
PLCs are some of the most malignant tumors and the fourth-leading cause of cancer-related death worldwide [66]. The types of liver cancer include HCC, which accounts for the majority (70–85%) of PLCs, cholangiocarcinoma (CCC), and combined hepatocellular cholangiocarcinoma (cHCC-CCC), which exhibits features of both HCC and CCC [66]. Hepatoblastoma is a rare liver tumor that predominantly originates in early childhood; it is the most common pediatric liver malignancy [66,67]. The main etiologies of PLC include hepatitis virus infections, alcohol abuse, NAFLD, and aflatoxins [66,68]. With the development of vaccinations and antiviral treatments, the major cause for PLC has gradually shifted from viral infections to NASH, in correlation with the growing global obesity pandemic [69,70].
Conventional treatment options for liver cancer patients include surgical resection, radiofrequency ablation, liver transplantation and multiple-kinase inhibitors such as, Sorafenib and Lenvatinib [71]. Unfortunately, the median progression-free survival time remains less than six months for most of these treatments [72]. With the emerging success of immunotherapy in targeting advanced-stage lung cancer and melanoma, immune checkpoint inhibitors have also been explored to treat liver cancer [73]. Currently, the combination of antibodies blocking PD-L1 and VEGF is the 1st line of treatment for HCC patients with unresectable tumours. However, the available data from clinical trials showed that less than half of the patients responded to the treatment [74].
Liver cancer manifestation is highly complex and involves the dysregulation of multiple genes and signaling pathways as the disease progresses [75]. Comprehensive genome and transcriptome profiling studies have been conducted to reveal the genetic landscapes of cancer cells and unravel the mechanisms of HCC initiation and progression [76-78]. Mutations in genes such as TP53, CTNNB1, CDKN2A, and TERT have been commonly identified in different patient cohorts [78-80]. Various underlying diseases and etiologies can induce tumorigenesis in the liver. Postinfection, the HBV genome potentially integrates with host cells and activates genes relevant to the cell cycle and proliferation such as TERT [81-83], stabilizing the telomeric ends of chromosomes to support unlimited cell expansion. Diseases such as NAFLD and long-term drug and alcohol abuse induce chronic inflammation in the hepatic niche [84]. Constant exposure to inflammatory cytokines promotes genetic insults that lead to unique gene mutations and epigenetic profile changes that favor cell survival under harsh conditions [85,86]. The interplay among those different disease etiologies creates an environment that promotes oncogenic transformation, clonal evolution, and the expansion of diverse cancer-initiating cells. Thus, liver cancers, such as HCC, are reported to exhibit significant interpatient and intratumoral heterogeneity.
For decades, researchers have derived 2D monolayer cancer cell lines from tumors and created mouse models to understand the disease and search for therapeutic targets. These immortalized cell lines are highly expandable at low cost, enabling high-throughput drug screening applications. However, a single immortalized cancer cell line only partially captures the tumor profile and constantly acquires new mutations during culture [8,40]. The lack of intratumor heterogeneity produces an inaccurate reflection of patient responses when such cell lines are used for drug screening. Moreover, a monolayer cell culture limits cell–cell interactions to a horizontal plane, and the nutrient and oxygen gradients observed in tumors are absent [8]. A study by Takai et al. [87] demonstrated that immortalized HCC cell lines cultured in 3D produce structural features resembling the glandular epithelium observed in vivo, and exhibit they increased resistance to chemotherapeutic agents. The cells in 3D also showed increased sensitivity to the TGF/β-induced epithelial–mesenchymal transition. Correspondingly, after engraftment in mice, the cells from the spheroids formed tumors more efficiently that metastasized more frequently than the cells cultured in 2D. Such studies highlight the advantages of alternative 3D in vitro models of PLCs. The advent of organoid culture thus presents a long-sought alternative strategy for creating human PLC models (Fig. 2). Commonly used immortalized cell lines have been derived and grown with undefined serum-supported media in monolayer cultures [88]. Although such serum-based culture conditions have enabled the creation of widely used immortalized cell lines, they fail to recapitulate the interpatient and intratumor heterogeneity observed in HCC tumors [89]. In contrast, organoid culture platforms use a chemically defined nutrient- and growth factor (cytokines and small molecules)–enriched medium that potentially supports the growth of various cancer cell clones. Another critical advantage of the organoid platform is the ability to culture both cancerous and healthy tissue from the same patient in parallel, which creates opportunities to study tumor development without interfering with individual genetic backgrounds (Fig. 2) [35]. The liver cancer tumoroid platform has contributed significantly toward understanding cancer cell biology, and it opens new possibilities for precision treatment in the clinic (Table 2) [8,90,91].

Tumoroids from primary liver cancers. Liver cancer tumoroids can be derived from the major liver cancer subtypes: hepatocellular carcinoma (HCC), cholangiocarcinoma (CCC), combined hepatocellular-cholangiocarcinoma (cHCC-CCC), and hepatoblastoma (HB). ICO culture enables the parallel culture of non-tumor organoids from adjacent tissue. The non-tumor organoids form large transparent cysts with thin cell layers, unlike the tumoroids, which are dense in structure with invaginating layers of cells. Patient-specific tumoroids can also be co-cultured with patient blood–derived or tumor–derived immune cells for mechanistic studies and the evaluation of immune-drug responses. These organoids provide a platform for the mechanistic study of primary liver cancer (PLC) initiation and progression and the future development of precision medicine.
Tumoroid models for PLC
Large PLC cohort studies across different ethnicities have reported significant interpatient diversity [92,93]. Therefore, building a patient-specific model to support a personalized treatment strategy is crucial for the effective treatment of this disease. The landmark study by Broutier et al. [35] demonstrated the potential of the organoid platform to achieve that breakthrough. That team showed that the ICO culture system could derive tumoroid cultures from surgical tumor resections of HCC, CCC, and cHCC-CCC [36]. Tumoroids isolated from the surgical resections preserved the mutational profile, transcriptome, and histological features of the original tumor, even after long-term (months) culture. Importantly, a proof of concept study further demonstrated the use of those organoids to 1) identify potential prognostic markers by comparing the gene expression profiles of tumoroids to ICOs generated from adjacent nontumor tissue and 2) evaluate the tumor subtype and patient-specific drug response. A parallel effort by Saltsmann et al. [67] further showed that the platform can derive tumoroid models from hepatoblastoma, for which human models have been scant. In another follow-up study, Nuciforo et al. [89] demonstrated that the culture platform could also work with needle biopsy samples and their much smaller tumor tissue volumes. That achievement could be essential for the use of future precision therapeutics, for which tumoroids could serve as patient avatars for in vitro screening to identify the most suitable treatment options [89]. To use the tumoroid platform to capture intratumor heterogeneity, Li et al. [94] divided a tumor into multiple sections and generated tumoroids for each section. In that way, 27 tumoroid lines were obtained from 5 HCC and CCC patients, and the responses to 129 cancer drugs were assessed. The drug response study revealed significant intratumoral functional heterogeneity in the tumoroids derived from different tumor sectors. In addition to human tissue, ICO organoid cultures can be used with GEMMs in mechanistic studies of HCC development. Cao et al. [95] used tumor organoids isolated from reporter mouse models to study the function of LGR5+ cells in tumor initiation. The LGR5+ cells expressed genes associated with multiple hepatic stem cell–related pathways, including the Wnt signaling, Notch signaling, and BMP signaling pathways. Those cells exhibited an increased capacity for tumoroid formation in culture and tumorigenesis in mice and increased resistance to conventional anticancer therapy compared with the controls.
In addition to tumoroid cultures, organoids derived from healthy liver tissues can also be used to model the development of liver cancer. Using human ICOs derived from healthy tissues, Artegiani et al. [96] revealed how BRCA1 associated protein-1 (BAP1) potentially functions as a tumor suppressor in ductal cells. Frequent mutations in the BAP1 gene have been reported by exome profiling studies of CCC patients [97]. However, the exact mechanism by which the chromatin modifier affects tumor initiation or progression remains unclear. Those authors demonstrated that BAP1 depletion results in the loss of epithelial cell identity in p53-depleted cholangiocyte organoids. Specifically, the organoid morphology transitioned from translucent cystic spheroids to dense organoids with thickened cell layers and invaginations, resembling tumoroids derived from PLC. BAP1 was found to modulate that phenotypic change via the transcriptional regulation of genes important for junction formation and cytoskeletal components. That study highlighted the immense potential of healthy organoids as an in vitro platform to validate the potential tumor suppressors and oncogenes identified in many PLC cohort studies. Using a similar approach, Yang et al. [98] were able to model YAP1-induced hepatoblastoma development with hepatocyte organoids (HOs) generated from healthy fetal livers. The HOs derived from fetal livers resembled hepatoblasts with high AFP expression. Those authors demonstrated that YAP1 activation is sufficient for oncogenic transformation. The process likely involves epigenetic changes driven by the histone methyltransferase G9a, and inhibitors targeting that molecule were shown to be promising therapeutics for YAP1-driven hepatoblastoma.
The abovementioned studies mainly used the tissue-derived organoid culture method pioneered by Hans Clevers and Meritxell Huch [28,47,99]. Taking a different approach, Sun et al. [100] generated HCC tumoroids using the transdifferentiation approach (Fig. 2). Hui’s group previously reported the successful transdifferentiation of fibroblasts to human-induced hepatocytes, and they found that the overexpression of the SV40 large T antigen enabled stable expansion [101]. By overexpressing c-Myc, a frequent HCC driver gene [102,103], those authors induced HCC in organoids that lost their hepatic functions and begin to form tumors when they were engrafted into immunodeficient mice. Using that platform, those authors revealed that Myc-driven HCC development likely involves dysregulated alterations in mitochondria-associated EndoReticulum (ER) membranes and mitochondrial functions. In addition, that team used the system to validate that human hepatocytes can be the cellular origins of CCC. Notably, the RAS-mediated CCC oncogenic transformation was evident only in the 3D organoid cultures, not their 2D counterparts, highlighting the value of modeling human diseases in organoid cultures.
Although organoid culture systems have enabled significant breakthroughs in modeling PLCs, several limitations remain. A major hurdle for adopting the ICO culture approach for precision medicine is the low tumoroid derivation efficiency that has been reported. The average success rate of ~30% reported in the abovementioned studies is much lower than the tumoroid culture efficacy achieved in other cancer indications, such as colon and pancreatic cancer [32,33]. Broutier et al. [35] reported that the low success rate is closely associated with the proportion of KI67+ proliferative cells in the biopsy. However, Nuciforo et al. [89] reported that tumoroids could only be derived from moderately and poorly differentiated tumors. Those results suggest that the culture of liver cancer organoids is limited to a proliferative and aggressive subset of cancers and might fail to capture the entire disease spectrum. Therefore the loss of cancer cell heterogeneity in the organoid culture may affect the in vitro prediction of clinical drug responses. Another concern is the presence of healthy organoids in tumoroid cultures. Essential growth factors should be removed from the culture in the first two weeks, and some of the healthy organoids must be mechanically removed from the tumoroid culture to reduce contamination [35]. However, long-term, stable tumoroid expansion still depends on using a complete growth factor medium that supports the culture of healthy organoids. This outgrowth of noncancer, healthy-organoid contaminants has also occurred in other tumoroid cultures that have used similar tissue-derived organoid culture approaches in lung [104], pancreas [33], and prostate [105] cancer research. Although treating tumoroid cultures with p53 pathway activators such as Nutlin3 can induce cell death in noncancer organoids, that strategy might not work for all cancer subtypes due to interpatient and intratumor heterogeneity. The future optimization of culture conditions will be critical to enabling the clinical use of precision medicine. A related issue in tumoroid culture is the clonal selection that occurs during long-term passaging. Although organoid culture potentially supports multiple cancer cell clones during derivation and early maintenance, that diversity is likely lost over time due to competitive growth between cancer clones. That process was reportedly observed in the Colorectal cancer (CRC) organoid biobank established by Fuji et al. [106]. Those authors co-cultured three pairs of CRC tumoroids, each pair derived from different regions of the same tumor. After 4–6 weeks, all three paired cultures were dominated by a single population of tumoroids. Although that loss of cancer cell heterogeneity was not investigated in the abovementioned PLC tumoroid studies, this issue is likely to be pervasive across tumoroid cultures for various cancer indications.
VIRUS-DRIVEN LIVER DISEASES
The human liver is a highly vascularized organ that is exposed to many external pathogens in the blood [1]. Common pathogens that infect hepatocytes include malaria-causing Plasmodium parasites and the hepatitis virus [108]. In contrast to Plasmodium parasites, the hepatitis virus affects host cell function and integrity, inducing liver inflammation and injury. Chronic hepatitis can progress to end-stage liver cirrhosis or liver cancer, and viral hepatitis was estimated to cause 1.34 million deaths globally and HBV and HCV infections accounted for 96% of mortality reported [109].
Understanding how these viral proteins and host–virus protein interactions function in each essential step of the viral life cycle has enabled the discovery of therapeutics. In HBV therapy, nucleoside reverse transcriptase inhibitors (NRTIs), which target HBV polymerase to disrupt viral genome replication, are currently prescribed to suppress viral levels in the liver [110,111]. Drugs targeting the interaction between L-HBs and NTCP, such as the myristoylated peptide Myrcludex B, have also been shown to reduce viral infection in preclinical models and early clinical trials [112]. Although targeting many of those viral pathways has enabled the effective management of the disease in patients with few liver complications, a cure is still unavailable because viral covalently closed circular DNA (cccDNA) persists in the host cell as a mini-chromosome or is integrated into the host genome [113]. In stark comparison, HCV is now widely considered to be a curable disease thanks to a range of direct-acting antiviral (DAA) drugs that target various viral nonstructural proteins [114]. Combinations of DAA drugs now enable 80–95% of HCV patients to be cured.
In a previous review of HCV research breakthroughs that enabled the rapid development of therapies, in vitro modeling for HCV replication was demonstrated to play a crucial role [114]. The discovery that the overexpression of cDNA encoding various viral genotypes induces full viral replication in hepatic cell lines enabled the rapid investigation and screening of therapeutics targeting different parts of the viral life cycle [115,115]. In vitro models continue to offer researchers mechanistic insights into how the HCV virus infects the host [117]. Human hepatic cell models, each with advantages and limitations, have also been used for HBV infection studies. Immortalized cancer cell lines, such as HepG2 and Huh7, are easy to culture and proliferate rapidly. However, they do not support HBV infection due to a lack of endogenous NTCP expression, so exogenous expression of NTCP is used in those cell lines to solve that problem. Although the ectopic expression of NTCP in those cells has proved useful [118-120], immortalized and mutating cancer cells might have lost cellular components that regulate viral entry. In light of that concern, HepaRG, a tumor-derived hepatic progenitor cell line capable of generating NTCP-expressing HLCs, is increasingly being adopted for HBV modeling [121-125]. However, the limitations of HepaRG in HBV modeling include low infection efficiency (requiring a high infection dose of >1,000 GE/cell) and low levels of cccDNA [126].
Liver organoids for viral-hepatitis modeling
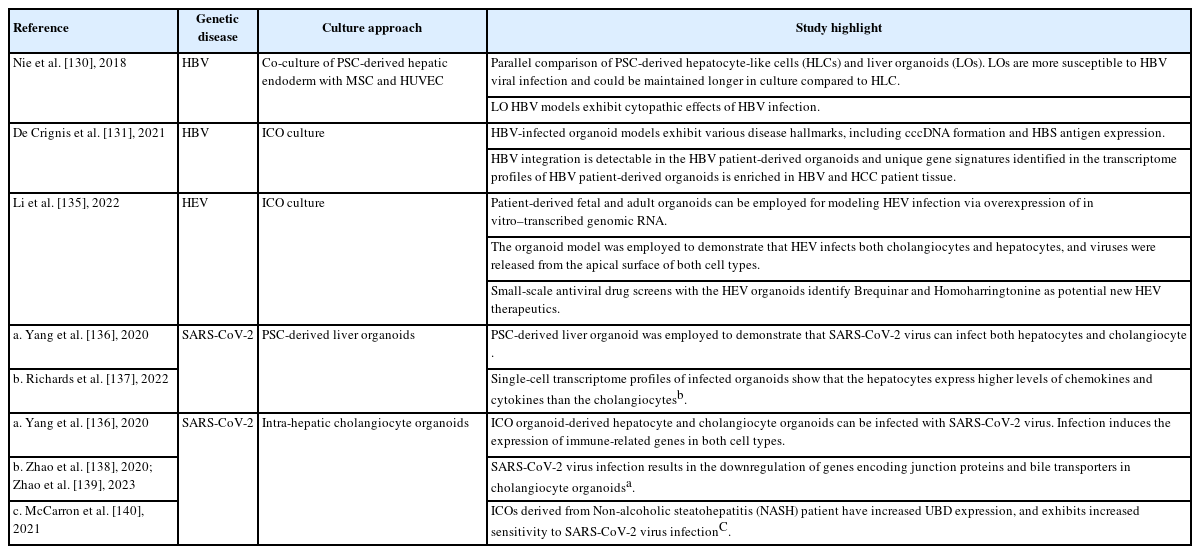
HLCs derived from PSCs are one source of renewable primary cells for modeling HBV infection [127-129], and multicellular organoids derived from iPSCs have also been explored (Table 3). Nie et al. [130] generated functional liver organoids (LOs) using the differentiation of PSC-derived endoderm cells co-cultured with mesenchymal stem cells and human umbilical vein endothelial cells. In that study, they demonstrated the advantages of modeling HBV with LOs, compared with HLCs generated from the same iPSCs. The LOs were shown to be more functional than their HLC counterparts, as reflected by the increased expression of cytochrome P450 genes, albumin and urea production, and CYP3A4 activity. Notably, although NTCP expression in the HLCs and LOs was comparable, HBV infection was much more efficient in the LOs, as reflected by the increased levels of pgRNA, intracellular viral DNA, and cccDNA. Correspondingly, a higher level of virion release was also detected in the LOs. Intriguingly, those authors discovered that LOs generated from different patient iPSCs demonstrated varying levels of HBV infectivity. Those results further support the role of host-specific factors in modulating HBV infection, which could account for variations observed within specific ethnic populations [130]. In addition, those authors demonstrated that the LOs could maintain their function in culture for up to 15 days longer than HLCs, enabling the evaluation of extended HBV infection. HBV-infected hepatocytes in the LOs generated virions that could further infect primary hepatocytes. Furthermore, the hepatocytes exhibited cytopathic responses, such as loss of hepatic function, injury marker expression, and structural changes, including increased cytoplasmic vacuoles and the loss of membrane microvilli. Although the existence of other cell types seemingly improves the hepatic functions of the LOs, it remains to be investigated whether the other cell types play a direct role in modulating viral infection. Further HBV modeling studies with multicellular organoids (Fig. 1) with different cellular compositions and structural features will advance knowledge about the roles of other liver cell types in HBV infection and progression.
The spectrum of patient profiles observed in HBV is diverse. Patient disease status ranges from individuals who have a high viral load in their blood but no hepatitis symptoms to individuals with hepatitis symptoms and a low viral load [110]. Such disparate patient responses indicate the need to develop patient-specific HBV models that address host factor–specific responses (Fig. 3). To that end, De Crignis et al. [131] used the ICO platform to successfully generate HBV organoid models derived from healthy and HBV-infected individuals. The organoid derivation efficiency was comparable from healthy and diseased tissues. The healthy organoids (HOs) and disease organoids (DOs) also exhibited similar proliferation and differentiation capacities. HOs infected with the HBV virus exhibited the hallmarks of infection, including cccDNA formation and viral antigen expression. Of note, the organoids further exhibited dose-dependent toxicity in response to NRTIs, which was not observed in HepG2 cells. This further supports the potential application of HBV patient–derived ICOs for testing idiopathic drug responses and toxicity. Those authors also demonstrated the amenability of the ICOs to genetic modification by creating transgenic cell lines that overexpressed NTCP and HBV virus–producing cell lines. Intriguingly, the NTCP-overexpressing ICOs cultured in expansion medium (nondifferentiated) did not exhibit greater HBV susceptibility than the differentiated ICOs. This observation further validates that hepatocytes need other factors to be susceptible to HBV infection, and it could account for the absence of detectable virus in ICOs derived from HBV patients. Nonetheless, those authors reported the detection of HBx coding region integration in 5 out of the 6 organoids assayed. In addition to viral replication, the HBx antigen has been implicated in immune protection from host cells and HCC development [132]. Thus, those organoids will likely be helpful in gaining mechanistic insights into how the antigen functions and promotes those activities. Those authors also identified a set of genes that is enriched in DOs, compared with HOs. The DO gene signature is reflected in HBV and HCC patient tissues, so it could potentially serve as an early prognostic marker for HCC development.
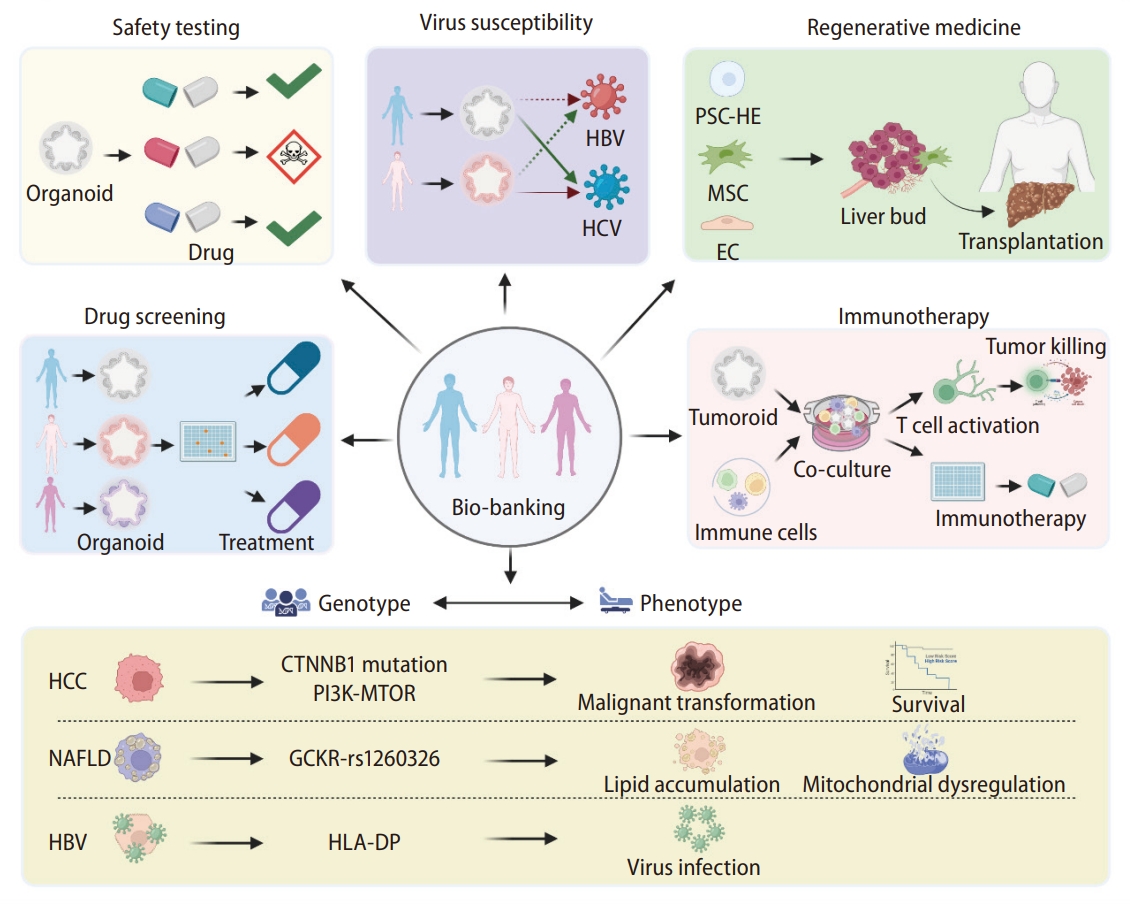
Patient-derived organoids for precision medicine. The organoid culture platforms have enabled researchers to generate patientspecific cellular models for precision applications, including various therapeutics and toxicity tests, regenerative therapy, and evaluations of organ susceptibility to viral infection. Accumulating such patient samples in large biobanks will enable genotype-to-phenotype correlation research and other pharmacogenomic studies. HBV, hepatitis B virus; HCV, hepatitis C virus; PSC, pluripotent stem cell; HE, hepatic endoderm; MSC, Mesenchymal Stem Cells; EC, endothelial cell; HCC, hepatocellular carcinoma; NAFLD, nonalcoholic fatty liver disease; HLA-DP, Major histone compatibility.
Although HEV infection is rare compared with HBV and HCV infection, the virus is known to infect pregnant people and have severe effects on maternal and fetal outcomes in resource-limited regions [133]. Unfortunately, available therapeutic options, such as antiviral ribavirin and pegylated interferon-α, are contraindicated during pregnancy [134]. Adopting the ICO organoid culture system, Li et al. [135] established fetal and adult liver organoid models of HEV infection to investigate potential therapeutic options. They demonstrated the feasibility of establishing HEV infection in the organoids and validated the broad virus tropism in cholangiocytes and hepatocytes and virus release in the apical surfaces of both cell types. In addition, they performed a small-scale drug screen with the organoids and demonstrated the potential of the antiviral homoharringtonine for HEV therapy. By manipulating the HEV variant introduced into the organoids, they further highlighted the utility of homoharringtonine in treating the ribavirin-resistant p6G1634R HEV variant [135].
Other virus-driven liver disease studies with organoid models
The major symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus infection are associated with the respiratory system. However, further clinical evidence and virus tropism studies suggest that the SARS-CoV-2 virus might infect cells in other organs, including the liver [136,141,142]. Importantly, a significant number of SARS-CoV-2 patients with severe symptoms were also reported to have abnormal liver enzyme levels [142,143]. To further understand how the liver could be affected by the SARS-CoV-2 virus, multiple groups have used the ICO culture [138,140] and PSC-derived organoid culture methods [136,137] to validate direct viral infection in hepatocytes and cholangiocytes, as well as to investigate disease manifestations in individual cells (Table 3). The discovery of ACE2 and TMPRSS as the proteins that mediate SARS-CoV-2 tropism prompted scientists to identify other potential cell types that the virus could infect by using singlecell tissue profiling experiments [144]. Analyzing single-cell profiles of liver tissue to identify SARS-CoV-2 target cell types has been challenging due to the low levels of ACE2 expression detected on a single-cell sequencing platform and the low percentage of cells expressing those proteins. Liver organoids generated from PSCs or tissue have helped to validate that the virus can infect both parenchymal cell types. Of note, a study by McCarron et al. [140] highlighted that NASH patient–derived organoids were particularly susceptible to SARS-CoV-2 infection due to the increased expression of ubiquitin D, which is an inhibitor of RNA virus–induced interferon signaling. This interesting finding indicates that direct virus tropism could contribute to the increase in abnormal liver functions and longer viral shedding time observed in NAFLD patients [145].
In summary, organoid platforms have been used to establish human models for various virus-driven liver diseases. These models have produced mechanistic insights about viral tropism and enabled the derivation of patient-specific models to address idiosyncratic responses to viral infections. The future growth of a biobank of hepatitis patient–derived organoids would likely accelerate the identification of hostspecific factors that modulate viral tropism and life cycle (Fig. 3).
NONALCOHOLIC FATTY LIVER DISEASE
NAFLD is a CLD with a high clinical incidence, and it is closely associated with other metabolic disorders such as obesity, diabetes mellitus, and metabolic syndrome [146,147]. Chronic inflammation and hepatic injury promote the progression of NAFLD to NASH and eventually cirrhosis and HCC [148]. The global prevalence of NAFLD in 2018 was ~24.4% [149], and correspondingly, the number of patients with end-stage cirrhosis and HCC due to NASH continues to rise [147]. Although NAFLD patients are clinically asymptomatic, and the condition is relatively benign, NASH is rapidly becoming the leading cause of end-stage liver disease and liver transplantation [150,151]. The high incidence of NAFLD is primarily attributed to diet and lifestyle changes. Irregular lifestyles, such as insufficient sleep and exercise, and the long-term intake of foods high in sugar and fats contribute to excessive fat accumulation in the liver [152]. Common metabolic diseases such as hypertension, hyperglycemia, obesity, and metabolic syndrome are risk factors for NAFLD and NASH development [153-155]. In parallel, genetics also likely play a role in NAFLD development. Familial studies have shown that children of parents with a high liver-fat content are more likely than others to develop NAFLD and have an increased risk of progression to cirrhosis [156]. Genome-wide association studies in multiple NAFLD cohorts have identified enriched single-nucleotide polymorphisms (SNPs) in various genes in NAFLD patients, including apolipoprotein C3, glucokinase regulator (GCKR), membrane-bound O-acyltransferase domain-containing 7, patatin-like phospholipase domain containing 3 (PNPLA3), and transmembrane 6 superfamily member 2 [157]. Furthermore, a protective allele that reduces the risk of NAFLD progression to NASH has been reported in the HSD17B13 gene [158].
NAFLD is a continuum of disease ranging from benign hepatic steatosis to NASH and cirrhosis [159], with 43–44% of patients with steatosis progressing to NASH, and 7–30% of patients with NASH progressing to cirrhosis [160,161]. The mechanisms underlying NAFLD progression have yet to be fully elucidated. A two-hit theory was first proposed for the pathogenesis of NAFLD [162]. The first hit involves lipid metabolism disorders and manifests as hepatic steatosis. That results in insulin resistance and leads to increased serum levels of free fatty acids (FFAs) and sensitizes the hepatocytes for the second hit, which comprises endoplasmic reticulum stress, oxidative stress, and mitochondrial dysfunction. Together, the two hits result in hepatocyte damage and the secretion of large amounts of proinflammatory factors that recruit and activate immune cells into a chronic inflammatory response [163]. Accumulating evidence has shifted that traditional two-hit model into a model of multiple, parallel pathogenic influences in which fatty acids, insulin resistance, inflammation, oxidative stress, mitochondrial dysfunction, lipid peroxidation, and endoplasmic reticulum stress combine to induce the onset and development of NAFLD [164]. In addition, gut microbes have been reported to be involved in the development of NAFLD via the regulation of bile acid metabolism, lipid handling, and endotoxin and metabolite production in the gut [165,166]. Chronic inflammation is central to NAFLD disease progression and involves both residential and circulating immune cells, including macrophages and lymphocytes [167,168]. Interactions among the different cell types also involve a slew of inflammatory chemokines and cytokines, such as chemokine ligand 2, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-1β, IL-17, and IL-18 [169-172].
Models of NAFLD have been critical for elucidating the mechanisms underlying this disease and identifying potential therapeutic targets. Multiple diet–induced models and GEMMs have been developed for NAFLD, and most of them recapitulate the histological features of NAFLD [173]. However, only a few studies have captured the metabolic syndromes commonly observed in patients [174], and there have been discrepancies in the NAFLD phenotypes observed in mouse and human models. In follow-up studies about PNPLA3 (the most widely reported NAFLD-associated gene), PNPLA3 depletion in mice did not affect hepatocyte lipid accumulation, which is in contrast to the findings in human in vitro models [175,176]. Human PNPLA3 levels are also much higher in liver tissue than adipose tissue, which is in contrast to rodent PNPLA3, which is more highly expressed in adipose tissue [177,178]. Given the increasing prevalence and lack of therapeutic options for NAFLD, many human models of NAFLD have been created using different hepatic cell lines and 2D and 3D culture platforms, including sophisticated microfluidics platforms [179] and PSC and tissue-derived organoid models (Table 4).
Modeling NAFLD with human liver organoids
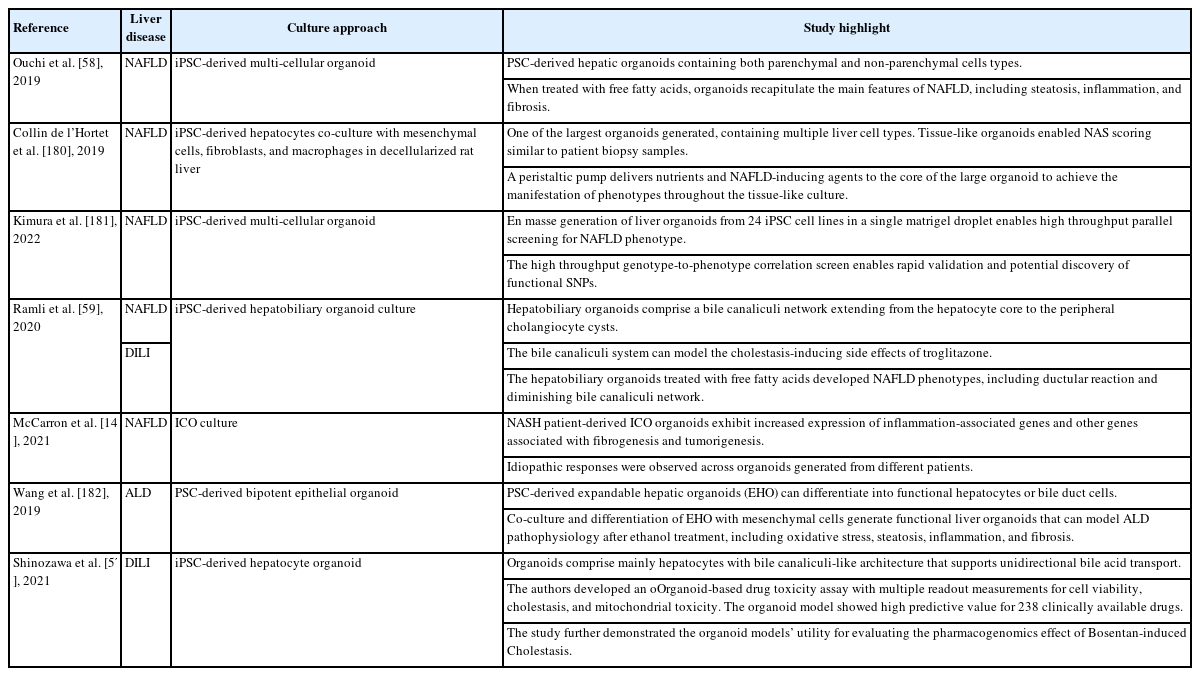
Although a fatty liver begins with the excessive accumulation of lipids in hepatocytes, disease progression involves multiple NPCs such as KCs and HSCs [171]. Those cells are central to the chronic inflammatory response and fibrogenesis that account for the hepatocyte cell loss and remodeling of the tissue architecture that occur as NAFLD progresses to NASH. The observed progression rate for NAFLD to NASH is ~14 years, and the progression rate in NASH patients is ~7 years per fibrosis stage [183]. This long time period highlights the complex interplay among different cell types that mediates NAFLD progression and the need to develop more advanced multicellular models. Ouchi et al. [58] reported one of the first PSC-derived multicellular organoids containing most liver parenchymal and NPC types, including HLCs, KCs, and HSCs. Although the cells in the organoids lacked a structural organization resembling that in tissue, the presence of cells resembling all the different liver cell types enabled the recapitulation of many NAFLD phenotypes upon treatment with FFAs. The triglyceride levels increased in the FFA-treated organoids, and the hepatocytes accumulated large lipid droplets and swelled. In addition, inflammatory responses were observed via the detection of cytokines such as TNF-α and IL-8. Furthermore, the FFA-treated organoids exhibited the hallmarks of fibrogenesis, including elevated P3NP levels, an increased number of α-smooth muscle actin–positive HSCs, and increased collagen deposition. Those authors also demonstrated that fibrogenesis in the organoids correlated with increased organoid stiffness, as measured by atomic force microscopy. Overall, that pilot study demonstrated the utility of multicellular organoids in modeling different phenotypes of NAFLD.
To capture the structural features of liver tissue, Ramli et al. used a stepwise PSC differentiation approach to generate hepatobiliary organoids with a functional bile canaliculi network [59]. Using a cocktail of palmitic and oleic acids, those authors also demonstrated the feasibility of generating NAFLD organoids in vitro that exhibit steatosis features and express transcriptome profiles resembling those of NAFLD patient tissues. The development of imaging techniques and analysis has revealed structural changes in the bile canaliculi during NAFLD progression [184]. Specifically, Segovia-Miranda et al. [184] reported that the bile canaliculi begin to narrow and shorten in NAFLD patients before NASH develops. Remarkably, the bile canaliculi network in the hepatobiliary organoid treated with FFA cocktails demonstrated a similar response. Future studies using these organoids will help provide mechanistic insights into those structural changes. Interestingly, those authors detected the proliferation of cholangiocyte cyst structures in the FFA-treated hepatobiliary organoids, in parallel with an increasing proportion of dying hepatocytes. This observation is similar to the ductular reaction (DR) observed in NASH patient tissues. However, the lack of NPCs in the organoids prevented further investigation of the DR-induced fibrogenesis observed in animal models [185]. Overall, that study demonstrated the potential of organoids to recapitulate structural changes observed in vivo.
The organoids described thus far are small (less than a millimeter) and not compatible with modeling larger structural features such as the liver lobules and vasculature. Hepatocytes in the liver lobules are distributed across zones with different levels of nutrients and oxygen [186]. It would be interesting to recapitulate those structural features to understand how liver diseases, including NAFLD, manifest differently across each lobule. To create a sizable physiological human liver model, Collin de l’Hortet et al. [180] used a co-culture system of iPSC-derived hepatic cells and NPCs in a decellularized rat liver scaffold. By using a peristaltic pump to introduce cells into the large tissue scaffold and continuously provide nutrients to all parts of the tissue-like culture, the group maintained the largest (centimeter-size) in vitro liver organoid to date. Notably, the vascular flow system was essential to achieve an NAFLD phenotype across the entire organoid. Compared with previous organoids, in which each NAFLD phenotype was assayed using a different molecular assay and multiple organoids, the achievement of a tissue-like organoid culture allowed those authors to perform histological NAFLD activity score (NAS) scoring, similar to the test used with tissue sections from patients. One key advantage of such a co-culture system, compared with the abovementioned stepwise differentiation approach, is the potential use of transgenic cells to study gene function in the cell type of interest. SIRT1 expression is downregulated in the hepatocytes of NASH patients, and SIRT1 knockout in mouse livers induces NAFLD [187]. Using SIRT1-knockout iPSC cell lines to generate hepatocytes for organoid formation, Collin de l’Hortet et al. [180] achieved the specific depletion of SIRT1 in those hepatocytes. They thus revealed the role of SIRT1 in regulating lipolysis and de novo lipogenesis in hepatocytes, and they demonstrated that SIRT1 depletion induced an NAFLD phenotype in the organoid. In follow-up studies, it would be interesting to explore whether that system can recapitulate hepatocyte zonation across large organoids and be further refined to achieve bidirectional sinusoidal and bile canaliculi flow. In particular, that study highlights the value of integrating bioengineering techniques to generate advanced liver organoid cultures. An organ-on-a-chip culture that uses microfluidics techniques is also frequently harnessed to create 3D liver models [188]. Microfluidics platforms enable the precision control of media flow and cellular placement to create cellular interactions and spatial organizations that resemble the liver architecture. Furthermore, integrating nanosensors and electronics in the chip enables researchers to monitor cellular changes during disease modeling. We expect the increasing use of such microfluidics platforms for organoid cultures to generate more complex 3D liver models for various applications [189].
The high failure rate observed in NASH clinical trials remains a crucial factor in the lack of approved therapeutics for NASH patients [190]. Challenges for drug development include the complex interactions of multiple disease drivers and the need to correct metabolic dysregulation and reverse fibrotic scars, which are likely driven by different mechanisms. Another factor that probably contributes to the high failure rate, as well as future challenges in prescribing an approved drug, is the influence of patient genotype on drug efficacy. That became evident in the early phase 2 trials of obeticholic acid. This drug was proved effective in a Western population but failed in a trial with a Japanese population [191,192]. Therefore, patient-specific models are needed to further identify the patient genotypes that modulate NASH development and evaluate patient-specific responses to NASH drugs. The further accumulation of these patient models into a biobank of comprehensive clinical, molecular, and phenotype profiles will enable the future correlation of patient genotypes and phenotype responses for pharmacogenomic applications (Fig. 3). Both iPSC-derived and tissue-derived organoid systems are potentially suitable for this application.
In a proof of concept study, Kimura et al. [181] generated NAFLD organoid models from 24 patient-specific iPSC cell lines to identify patient-specific genotypes that correlate with NAFLD phenotype development. They established a high-throughput en masse differentiation platform that they termed the Population organoid platform to enable the parallel culture of 24 patient organoids in a single Matrigel droplet and the induction of an NAFLD phenotype. Using a combination of imaging and sequencing readouts, those authors mapped NAFLD phenotype development in the organoids to specific patient genotypes. They demonstrated the utility of their platform by validating the contribution of the GCKRrs1260326:C>T SNP to hepatocyte lipid accumulation via mitochondrial dysregulation. In addition, a retrospective evaluation of clinical studies revealed that that variant contributed to a higher risk of NASH development only in diabetic patients. Furthermore, with the available patient models for the targeted genotype, they were able to identify drugs that could reverse the mitochondrial dysregulation induced by the GCKR variant. Overall, this study lays a strong foundation for the future pharmacogenomic evaluation of NASH therapeutic drugs. This evaluation will likely aid in the identification of patients with genotypes that are suitable for specific drug trials or the exclusion of individuals at high risk for adverse drug responses. Such achievements will significantly accelerate the approval of new drugs for NASH therapy. In a parallel study, McCarron et al. [140] successfully generated long-term expanded ICO organoids from tissue obtained from multiple NASH patients. The organoids from NASH patients exhibited significant upregulation of pro-inflammatory and cytochrome p450–related pathways and liver fibrosis markers. Notably, the upregulation of different markers is idiopathic and not observed across the board. This achievement supports the potential use of tissue-derived organoids for setting up population-specific patient organoid biobanks for precision medicine studies.
ORGANOID MODELS FOR DRUG-INDUCED LIVER INJURY AND ALCOHOL-RELATED LIVER DISEASE
DILI is a common cause of liver injury and a significant challenge for drug development. Statistically, nearly one-third of drug candidates are withdrawn from the clinic due to adverse drug reactions [193]. Therefore, preclinical drug safety studies are critical to drug development. Drug toxicology studies rely heavily on animal testing in Investigational New Drug applications. However, animal model results have limitations due to significant species-specific physiological differences and the long latency period of some drug-induced injuries [194]. PHHs remain the preferred human in vitro model for DILI testing. The methods used to assess DILI with PHHs are currently limited to the measurement of hepatic enzyme activity, cell structural changes, and cell redox status [195,196]. Therefore, more sophisticated liver models are needed to allow researchers to test for changes in human liver tissue features. Shinozawa et al. [57] reported an iPSC-derived hepatocyte organoid model for high-throughput toxicity screening. The hepatocytes in the cyst-like organoid exhibited polarity and unidirectional transport of bile into the lumen. Using those organoids, those authors set up a screening platform that allows the real-time imaging of bile transport and parallel assays for cell viability. They validated 206 clinically available drugs with reported DILI and showed a high predictive value of ~90% sensitivity and specificity. By treating the organoids with FFA, those authors also demonstrated that the system could be used to investigate DILI under various liver disease backgrounds. In the abovementioned hepatobiliary organoid generated by Ramli et al. [59], the authors demonstrated how it could be used to model cholestatic events in DILI. They performed live-imaging of the organoids with 5(6)-carboxyfluorescein diacetate (CDFDA), which is a fluorescent probe that is transported in the bile system. Treatment of the organoids with the cholestasis-inducing troglitazone resulted in a gradual loss of signals in the bile canaliculi network and the accumulation of CDF (de-esterified CDFDA) in the enlarged cells. Overall, both studies highlight how researchers can harness the structural features observed in organoids to model DILI. We expect that toxicological screening applications will increase in the future with the generation of organoid models that can recapitulate more complex structural features of the liver.
In addition to DILI, alcohol abuse is a common cause of liver morbidity and mortality. Globally, approximately 3 million people die each year from alcohol consumption, and 8–20% of heavy drinkers develop cirrhosis [197]. Similar to NAFLD, ALD phenotypes include steatosis, fibrosis, and cirrhosis [223]. A study by Wang et al. [182] explored the use of PSC-derived hepatic organoids to recapitulate the pathogenesis of ALD. They first derived an expandable hepatic progenitor organoid that the enabled long-term expansion and generation of functional cholangiocyte or hepatocyte organoids. Subsequently, they achieved a co-culture of fetal mesenchymal cells and hepatocyte organoids. The organoid expressed ethanol (EtOH) metabolism–associated enzymes such as alcohol dehydrogenase and CYP2E1. When they were treated with EtOH, the cells exhibited increased cellular stress, steatosis, and a proinflammatory response. In addition, the authors detected elevated expression levels of fibrogenesis-related proteins, such as alpha-smooth muscle actin, type 1 collagen, and desmin. In summary, PSC-derived hepatic organoids can also be used to model ALD, but the utility of this model for mechanistic studies and the identification of novel therapeutics remains to be explored.
CONCLUSION
Liver organoids have provided researchers with an alternative human in vitro model that harbors features of in vivo tissue. Although these models encompass more physiological characteristics of the human organ than monolayer hepatocyte models, organoids should not be considered as a replacement for animal models, at least not in their current state. Organoids still lack many structural features of the tissue, a functional vascular system, and circulating hemopoietic cells. Future improvements to cell culture reagents and tools and the integration of bioengineering platforms will likely bring significant breakthroughs. Nonetheless, the current organoid culture platforms have already produced several advances in modeling human liver diseases. In particular, the ICO platform has enabled the generation of patient-specific liver organoid models for all the conditions discussed. This breakthrough will allow the generation of patient-specific models for various applications, from evaluating host susceptibility to viral infection to liver regenerative therapy (Fig. 3). In the next decade, we expect to see the establishment of sizeable organoid biobanks for various liver diseases. These biobanks will greatly facilitate pharmacogenetic studies by correlating patient genotypes to disease phenotypes and patient drug responses (Fig. 3), laying the foundations for precision therapeutics for treating liver disease.
Notes
Authors’ contribution
Y. Liu, J.Y Sheng, C.F. Yang and Y.S Chan reviewed articles and wrote the manuscript. Y.S Chan and J.J Ding oversee the review conception and writing.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
Figures 1 to 3 were created with BioRender.com.
Abbreviations
NPCs
nonparenchymal liver cells
HSCs
hepatic stellate cells
KCs
Kupffer cells
DILI
drug-induced liver injury
CLD
chronic liver disease
PLC
primary liver cancer
NAFLD
nonalcoholic fatty liver disease
ALD
alcoholic liver disease
ECM
extracellular matrix
PSC
pluripotent stem cell
iPSC
induced pluripotent stem cell
PHHs
primary human hepatocytes
HCC
hepatocellular carcinoma
ICOs
intrahepatic cholangiocyte organoids
A1AT
a1-antitrypsin
ALGS
Alagille syndrome
GEMMs
genetically engineered mouse models
CLC
cholangiocyte-like cell
CF
cystic fibrosis
CFTR
cystic fibrosis transmembrane conductance regulator
HLCs
hepatocyte-like cells
AFP
alpha-fetal protein
CCC
cholangiocarcinoma
cHCC-CCC
combined hepatocellular cholangiocarcinoma
HBV
hepatitis B virus
HCV
hepatitis C virus
NASH
nonalcoholic steatohepatitis
HOs
hepatocyte organoids
NRTIs
nucleoside reverse transcriptase inhibitors
NTCP
sodium taurocholate co-transporting peptide
DAA
direct-acting antiviral
LOs
liver organoids
HOs
healthy organoids
DOs
disease organoids
SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
SNPs
single-nucleotide polymorphisms
GCKR
glucokinase regulator
PNPLA3
patatin-like phospholipase domain containing 3
FFAs
free fatty acids
TNF-α
tumor necrosis factor-alpha
IL-6
interleukin-6
DR
ductular reaction