Neuropilins as potential biomarkers in hepatocellular carcinoma: a systematic review of basic and clinical implications
Article information
Abstract
Hepatocellular carcinoma (HCC) is one of the most common and deadly cancers worldwide and is characterized by complex molecular carcinogenesis. Neuropilins (NRPs) NRP1 and NRP2 are the receptors of multiple proteins involved in key signaling pathways associated with tumor progression. We aimed to systematically review all the available findings on their role in HCC. We searched the Scopus, Web of Science (WOS), PubMed, Cochrane and Embase databases for articles evaluating NRPs in preclinical or clinical HCC models. This study was registered in PROSPERO (CRD42022349774) and include 49 studies. Multiple cellular and molecular processes have been associated with one or both NRPs, indicating that they are potential diagnostic and prognostic biomarkers in HCC patients. Mainly NRP1 has been shown to promote tumor cell survival and progression by modulating several signaling pathways. NRPs mainly regulate angiogenesis, invasion and migration and have shown to induce invasion and metastasis. They also regulate the immune response and tumor microenvironment, showing a crucial interplay with the hypoxia response and microRNAs in HCC. Altogether, NRP1 and NRP2 are potential biomarkers and therapeutic targets, providing novel insight into the clinical landscape of HCC patients.
INTRODUCTION
Liver tumors are common and deadly cancer, with increasing incidence and mortality rates [1]. Hepatocellular carcinoma (HCC) is the main primary liver tumor type, accounting for approximately 90% of all cases [2]. HCC is a heterogeneous cancer mainly diagnosed at advanced stages. Its survival rate remains very low despite the available systematic therapies that only increase the survival probability of patients 1–2 years [2,3]. It is characterized by unique molecular carcinogenesis involving multiple modulators, signaling pathways, and mechanisms [2,4,5]. All of these factors contribute to the difficulty in understanding HCC development and progression. Therefore, further studies are required to provide clearer insights into its molecular mediators to develop effective targeted therapies and improve the outcomes of HCC patients [2,4].
The molecular pathogenesis of HCC is generally complex, with increasing numbers of molecules reported to participate in the development, progression, and drug sensitivity of HCC cells [2,6]. Neuropilins (NRPs) have recently been the focus of several studies due to their involvement in numerous cellular processes in cancer, such as cell proliferation, migration, invasion, and angiogenesis [7,8]. NRPs are 130–140 kDa type-1 membrane glycoproteins [7] with extracellular, transmembrane, and cytoplasmic domains. They act as co-receptors for proteins such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and transforming growth factor β (TGF-β) [7,9]. There are two NRPs (NRP1 and NRP2) encoded by different genes on independent chromosomes but with a common domain structure [8,9]. While both NRPs share some characteristics and ligands, they differ in their tissue distribution and modulated signaling pathways [8,10]. Moreover, while NRP1 has been widely studied and characterized, fewer studies have focused on NRP2 [9]. Nevertheless, the role played by both NRPs in cancer, including HCC, has recently become of interest due to their strong correlation with key cellular and molecular mechanisms involved in tumor progression [8,9].
Interestingly, despite increasing studies evaluating the role of NRP1 and NRP2 in human HCC, no attempt has been made to compile the main findings of their potential roles in the HCC tumor landscape. Therefore, we aimed to summarize through a systematic literature review the findings of published studies using preclinical and/or clinical HCC models and evaluating one or both NRPs.
MATERIALS AND METHODS
Protocol and registration
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [11] (Supplementary Tables 1 and 2) and was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD4 2022349774).
Literature search strategy
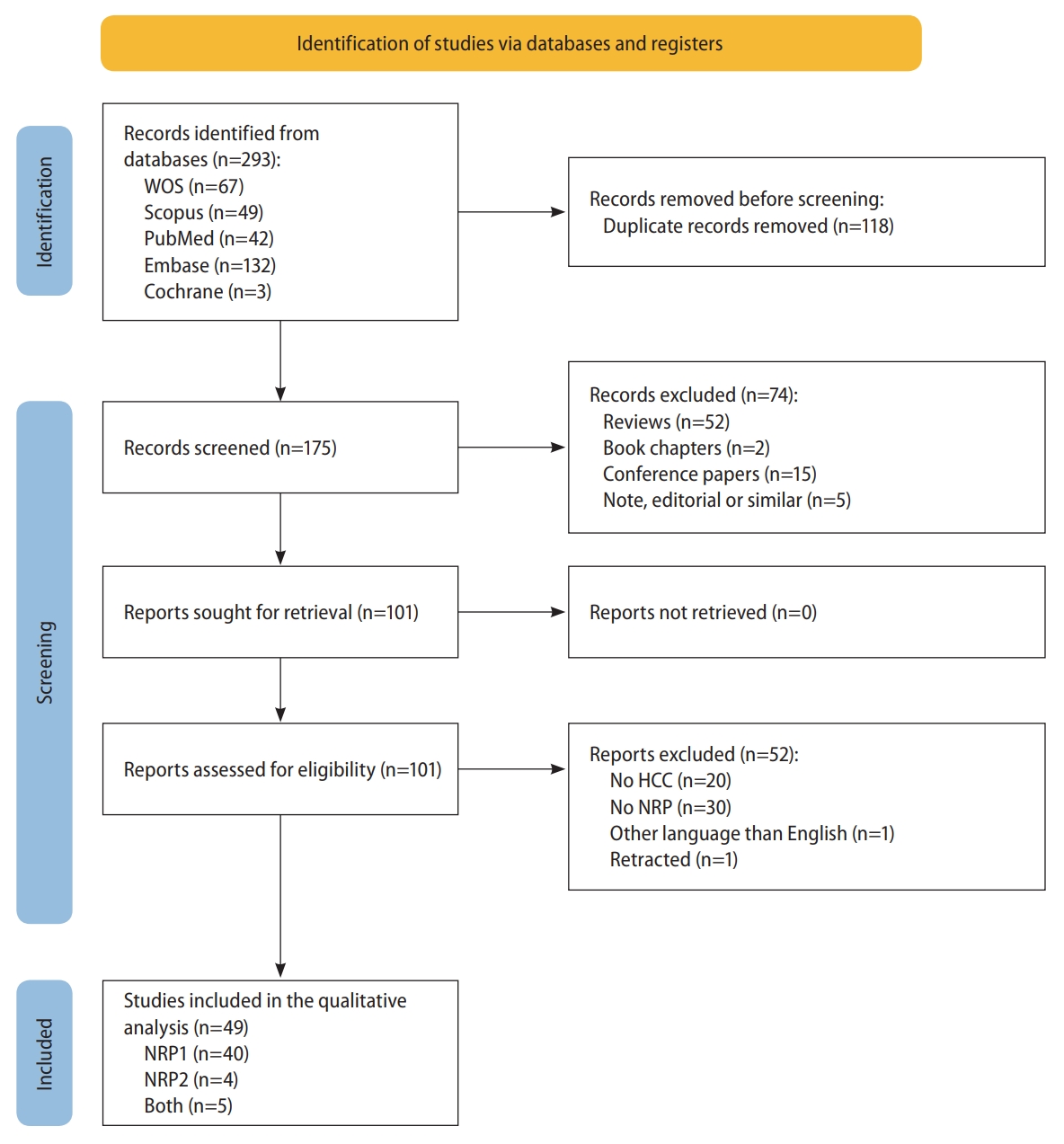
A comprehensive literature search was performed in the Web of Science, Scopus, PubMed, Embase and Cochrane Library databases of articles published up to August 31, 2022, identifying 293 articles (Fig. 1). This search strategy combined the search terms “NRP”, “NRP1”, “NRP2”, and “HCC”, in the search queries used for each database (Supplementary Table 3).
Inclusion and exclusion criteria
Studies that met the following eligibility criteria were included in this systematic review: (1) they involved human patients diagnosed with HCC, animal HCC models, or in vitro primary or genetically-modified HCC cell line models; (2) they determined NRPs expression or derived effects from modifying NRPs; (3) they evaluated tumor-associated processes or characteristics. Studies that met the following exclusion criteria were removed during the study selection process: (1) they involved human patients without an HCC diagnosis, or unsuitable or poorly-described HCC models; (2) they were reviews or similar articles; (3) they did not evaluate or report NRPs expression or derived effects from modifying NRPs; (4) their full-text was not available in English.
Study selection
Two authors independently performed the study selection process, and discrepancies were resolved by a third author based on a consensus.
Duplicates among the original articles identified in the initial search of all databases were removed. Next, the articles were subjected to an initial screening by title and abstract based on exclusion criteria. Then, the remaining articles’ full texts were screened against the eligibility criteria, and those meeting the inclusion criteria were identified and included in the qualitative analysis.
Data collection and analysis
Three authors independently extracted data from the included studies, compiling the information in separate tables for preclinical and human studies.
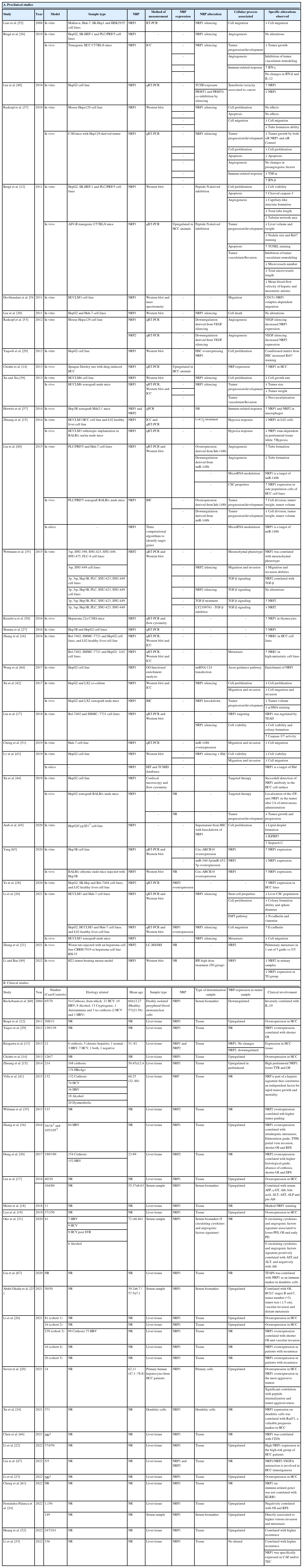
The following information was included from preclinical studies: the first author’s name, publication year, model used, sample type, NRP subtype, measurement method, NRP alteration, associated cellular process, and alterations observed.
The following information was included from human studies: the first author’s name, publication year, case and control number, related etiology, mean age, sample type, NRP sub-type, determination sample type, tumor tissue expression, and clinical involvement.
RESULTS
Study selection and characteristics
The complete literature search and study selection process was conducted as described in Figure 1. The comprehensive search identified 293 unique articles after removing 118 duplicates between the five databases. Both inclusion and exclusion criteria were used to select studies that fit this study’s scope, identifying 49 eligible articles for inclusion in the systematic review.
The number of published articles assessing the NRPs’ role in different HCC models has increased in recent years, with most studies conducted since 2010 (Supplementary Fig. 1A). Curiously, in vitro studies have been replaced by in vivo studies using animal models or human samples. Moreover, when separately considering published studies on HCC patients (Supplementary Fig. 1B) and animals (Supplementary Fig. 1C), we observed an appreciable increase in published studies involving human patients in the last three years, along with increases in the number of patients used (Supplementary Fig. 1B). However, while in vivo models have been constantly used over time, their sample sizes have increased in recent years (Supplementary Fig. 1C). The main characteristics of all included articles are summarized separately for studies involving preclinical models (Table 1A) and human HCC samples (Table 1B).
All 49 of the included studies evaluated the role of NRP1 and/or NRP2 in HCC, of which 11 used only in vitro models (22.45%), four only animal models (8.16%), seven both in vitro and in vivo models (14.29%), 19 only human HCC patient samples (38.78%) and eight preclinical and clinical samples (16.33%). There was a notable difference in the number of studies examining each NRP subtype. Only four of the 49 included studies examined NRP2 (8.16%), compared to 40 that examined NRP1 (81.63%), five examined both NRPs (10.20%). Most (22/27 [81.48%]) clinical articles on human patients evaluated NRP protein expression directly in tumor tissue. While detecting potential tumor markers in serum samples is an important tool for improving HCC diagnosis, only five studies analyzed serum NRP1 levels in human patients.
NRP measurements in preclinical models used different approaches. The most common was the real-time reverse transcription polymerase chain reaction (qRT-PCR; 33.33%), followed by western blot (16.67%), qRT-PCR and Western blot (20.00%), immunofluorescence microscopy (6.67%), and all three techniques (10.00%). In addition, some individual studies used different methodologies including qRT-PCR and immunofluorescence microscopy (3.33%), western blot and immunofluorescence microscopy (3.33%), and mass spectrometry (3.33%). One of the 30 preclinical studies did not assess NRP expression (3.33%).
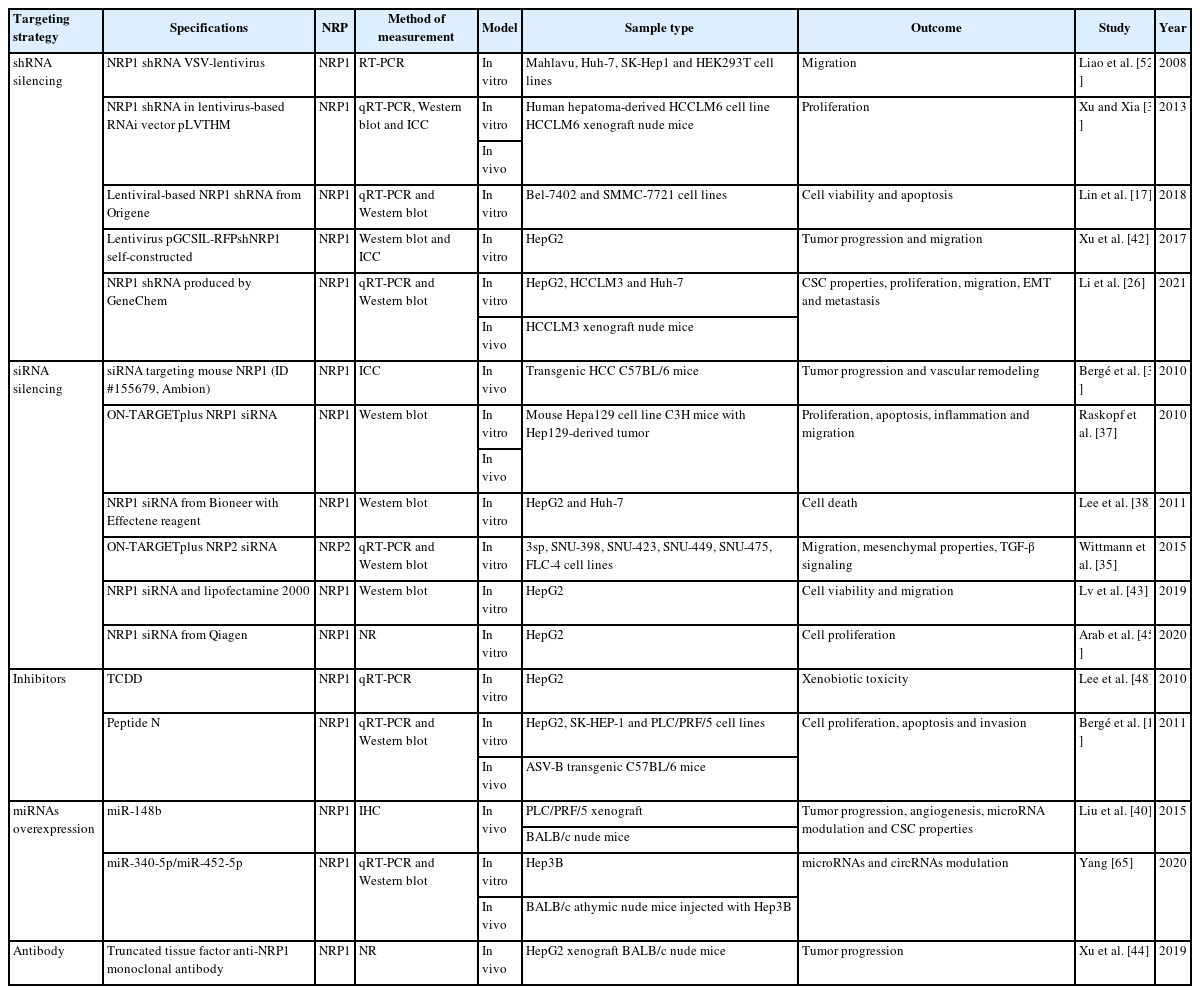
In addition, most included studies targeted one NRP to assess the derived effects. However, their targeting strategies varied widely, from genetic silencing to using inhibitors or antibodies (Table 2). Given the many articles evaluating the NRPs’ role in various cellular and molecular processes in HCC, their main findings have been organized and described in the following sections.
NRPs as diagnostic tissue or serum biomarkers
While NRPs were initially identified in the nervous system as axon guidance regulators [10], recent studies have reported multiple molecular functions [7,10]. Late diagnosis is one of the leading causes of HCC patients’ high mortality rate due to the absence of highly sensitive and specific biomarkers [2]. Therefore, an increasing number of studies have focused on the potential use of NRPs as tissue or serum biomarkers evaluating their expression levels in tumor and healthy liver samples from HCC patients to improve the current diagnostic tools for HCC diagnosis [12–26].
Studies have determined NRP protein levels in tumor tissue from HCC patients, finding NRP1 to be overexpressed compared to healthy liver tissue [12,14,16-20,23,24,26] and in high-risk HCC patients [22]. In contrast, Kitagawa et al. [13] did not find a difference, while lower NRP1 levels in tumor compared to peritumoral tissue have also been reported [15]. Similarly, NRP1 levels were increased in three HCC cell lines compared to the normal liver L02 [26], and Hep3B and HepG2 [27]. Contrariwise, NRP2 expression in HCC tissue has been analyzed to a lesser extent. Lower NRP2 expression was found in the HCC nodules versus the healthy liver tissue [13]. However, contradictory results have been reported for NRP2. While its urinary levels were decreased in a rat HCC model [21], an in vitro study found it was overexpressed in three human HCC cell lines [28]. These find ings highlight the necessity of further studies to clarify NRP2’s exact role in HCC.
Early HCC diagnosis is one of the main objectives of current clinical studies, where identifying useful biomarkers is an exciting area [2]. While all tissue and serum biomarkers are valuable tools for diagnosis at any tumor stage, non-invasive methods are usually preferred [3]. Only two studies have analyzed the potential use of either NRP1 or NRP2 as serum biomarkers, reporting that NRP1 could be a useful protein for HCC diagnosis based on its elevated levels in serum samples from patients with advanced HCC [17,25].
NRPs as prognostic biomarkers
Consistent with previous evidence, numerous studies have evaluated the prognostic role of NRP1 and NRP2 in HCC patients [15,16,24-26,29-33]. NRP1 overexpression was significantly correlated with shorter overall (OS) [16,24-26,29] and recurrence-free survival (RFS) [16,24] survival in patients, while NRP2 overexpression correlated with their lower OS and disease-free survival [30]. Moreover, NRP1 expression was correlated with RAD51 in hepatic stellate cells (HSCs), a valuable prognostic biomarker [34], and with an increased recurrence rate [32,33]. Peritumoral NRP1 levels have also been evaluated in HCC human samples. Patients with higher peritumoral NRP1 expression had higher OS, higher time to recurrence, and lower early recurrence incidence [15]. Intriguingly, a serum signature of nine circulating cytokines and angiogenic factors (NRP1, VEGF receptor 3 [VEGFR3], FGF23, Fas ligand [FasL], HGF, VEGFD, interleukin 1 receptor 2 [IL1R2], platelet-derived growth factor-BB [PDGF-BB], and Met tyrosine-protein kinase [c-MET]), correlated significantly with OS, progression-free survival, and early progression disease in advanced HCC [31].
In addition, associations between NRP overexpression and other clinical characteristics related to tumor aggressiveness have been reported. Both NRPs correlated strongly with advanced HCC stages. However, the staging method used differed [16,35], with NRP1 correlating with Edmondson grade and TNM classification [16], and NRP2 correlating with higher tumor grading [35]. Curiously, several studies found no association between both NRPs and age, sex, tumor size, and hepatitis B virus (HBV) infection [16,24,30]. Nevertheless, NRP1 overexpression was positively correlated with alpha-fetoprotein and other liver function markers and negatively correlated with albumin (Alb) and pre-Alb [17]. Moreover, increased serum NRP1 levels correlated with advanced Barcelona Clinic Liver Cancer stages (B and C), a tumor number ≥3, and a tumor size ≥ 5cm [25], highlighting the potential roles of NRPs in prognosis and different tumor-associated characteristics in human patients with HCC.
NRP effects on tumor progression and associated signaling pathways
NRPs have been described as relevant oncology proteins due to their modulation of axon guidance and cell survival, migration, angiogenesis, and invasion [10]. This designation is directly associated with numerous articles evaluating their role on different cellular processes and molecular signaling pathways involved in HCC progression (Fig. 2) [12,15,17,26,35-48].
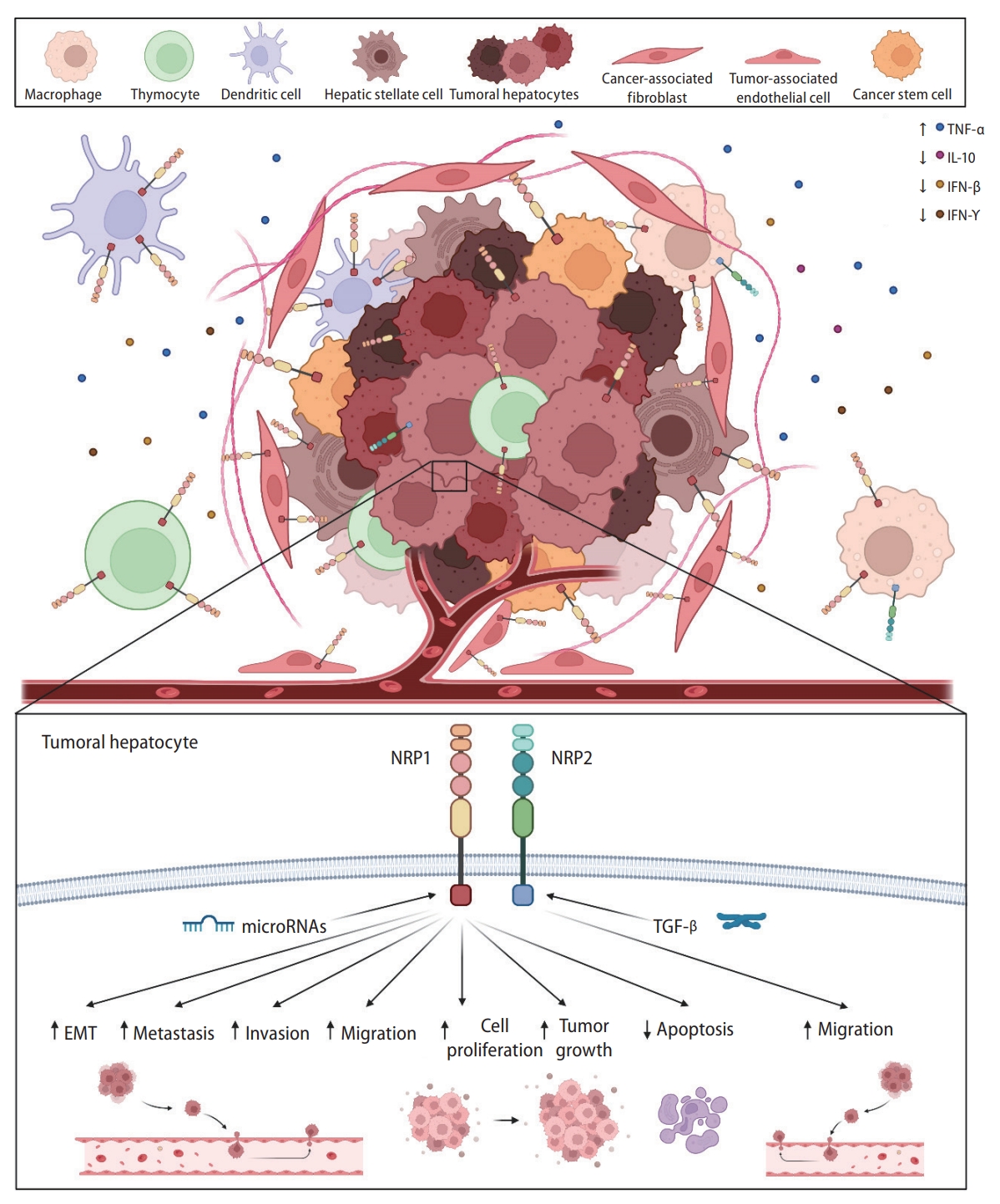
Main cellular and molecular mechanisms modulated by NRP1 and NRP2. NRPs are expressed in tumor cells and other tumor-associated populations that constitute the tumor microenvironment and participate in the immune response. Both NRP1 and NRP2 are expressed in a broad number of cell types and are involved in different cellular and molecular mechanisms responsible for HCC development and progression, modulating several cellular processes. EMT, epithelial-to-mesenchymal transition; IFN-β, interferon beta; IFN-γ, interferon gamma; IL-10, interleukin-10; NRP, neuropilin; TGF-β, transforming growth factor β; TNF-α, tumoral necrosis factor-α.
Both NRPs promote tumor progression by modulating cell proliferation, viability and apoptosis, with NRP1 being the most characterized. Numerous preclinical investigations showed that NRP1 downregulation decreased the growth and viability of HepG2 [12,42,43], SK-HEP-1 [12], PLC/PRF/5 [12,40], HCCLM6 [39], Huh-7 [26,40], Bel-7402, SMMC-7721 [17] and HCCLM3 [26] cells. However, no effects were observed in mouse Hepa129 cells [37]. Similar effects have been described in animal models, where a marked tumor growth inhibition was observed after NRP1 knockdown [12,36,37,39,42,44,49]. A five-gene signature that included NRP1 was identified as an independent risk factor for a faster tumor growth rate in HCC patients [41]. Furthermore, an interaction between NRP1 and VEGFA promoted HCC tumorigenesis by dysregulating cell-to-cell interactions in patients with HCC [47].
Similarly, apoptosis was altered when NRP1 expression was abolished in both in vitro and in vivo models, leading to an increase in cleaved caspase-3 levels and TUNEL-positive cells [12]. However, a previous study did not report cell death changes after NRP1 silencing in two HCC cell lines [38]. Peritumoral NRP1 expression was decreased and correlated with increased tumor size and other associated characteristics [15], highlighting NRP1’s crucial role in HCC tumor progression. In addition, NRP1 correlated positively with the immune marker cluster of differentiation 36 (CD36), a potential prognostic and immunologic marker in different cancers, including HCC [46]. Intriguingly, a recent study treated HepG2 cells with the supernatant from NRP1-downregulated HSCs, causing a decrease in lipid droplet content and insulin-like growth factor binding protein-3 (IGFBP3) expression, and an increase in serpin family A member 12 (SERPINA12) levels [45].
Only one study has analyzed the processes modulated by NRP2 [35]. Specifically, it was directly modulated by TGF-β signaling and correlated with a mesenchymal-like phenotype in vitro. Curiously, while TGF-β overexpression increased NRP2 levels, NRP2 silencing did not exhibit significantly altered TGF-β expression [35].
Role of NRP in migration and invasion-related processes
Invasion and migration processes are well-described events that characterizes the aggressiveness of solid tumors and are highly associated with tumor progression and recurrence [50].
The NRPs’ main effects in human pathologies are generally related to the modulation of signaling pathways that drive angiogenesis and cancer cell migration [10]. Numerous studies evaluated the NRPs’ role in these cellular processes in preclinical models [12,26,35-37,39,40,42,43,51-54] and human patients (Fig. 2) [24,25]. NRP2 downregulation significantly decreased the migration of two different HCC cell lines [35]. However, most studies have described similar results after NRP1 knockdown, finding lower cell migration ability in different cellular models, including the HCCLM3 [26,54], Huh-7 [26,51,52], HepG2 [42,43], Mahlavu, SK-Hep1, and HEK293T human lines [52] and mouse Hepa129 cell line [37].
NRP1 was also overexpressed in the highly metastatic Bel-7402 cell line, but was underexpressed in the less metastatic HepG2 and SMMC-7721 cell lines [16]. A recent study evaluated NRP1 function in metastasis, generating an HCC mouse model using NRP1-silenced and non-silenced HCCLM3 cells [26]. Curiously, they found that NRP1 downregulation greatly diminished the number of grafted mice with lung metastasis (1 of 5), compared to control mice (5 of 5) [26]. Similar findings have been reported in studies on HCC patients, where those with elevated NRP1 levels had a higher probability of venous invasion and metastasis [24,25].
At the molecular level, the NRPs’ role in angiogenesis-related signaling has been analyzed by two studies [26,53]. After VEGF silencing in the mouse Hepa129 HCC cell line, both NRP1 and NRP2 levels were markedly downregulated [53], supporting their interplay with the VEGF family ligands described in other tumor types [10]. Moreover, a recent study found that NRP1 modulated the epithelial-to-mesenchymal transition (EMT) pathway in two HCC cell lines, with decreased N-cadherin and vimentin expression and increased E-cadherin levels observed after NRP1 silencing [26].
Other relevant clinical aspects associated with tumor invasion and metastasis have been evaluated and associated with NRPs (Fig. 2) [12,36,37,39,40]. Two independent studies performed by the same research group successfully inhibited NRP1 activity through genetic silencing or inhibitor treatment, significantly reducing tumor vascular remodeling [12,36]. Moreover, an in vitro matrigel assay analysis of capillary-like structures showed that NRP1 inhibition with peptide N decreased the formation of capillary-like structures and other tumor-associated characteristics [12]. In this line, while the tubeforming ability of mouse Hepa129 cells decreased after NRP1 silencing [37]. NRP1 overexpression via microRNA (miRNA)-148b (miR-148b) silencing raised the tube-forming ability of human PLC/PRF/5 HCC cells [40]. Moreover, lower neovascularization was observed in an in vivo HCC mouse model after NRP1 silencing [39].
Studies on human HCC patients have described similar results. NRP1 overexpression was significantly correlated with intrahepatic metastasis [16] and vascular invasion [16,26] in two independent HCC cohorts, supporting the potential roles for NRP1 and NRP2 in the mechanisms underlying HCC progression.
NRPs and the immune response
HCC is a complex and heterogeneous tumor in which malignant hepatocytes and other tumor-associated cells affect the development, progression, and drug responsiveness of tumor cells [55,56]. Both innate and adaptive immune cells have been strongly associated with the modulation of cellular responses to chronic inflammation, fibrosis, or cirrhosis contributing to hepatocarcinogenesis and HCC progression [56]. Several investigations have focused on the tumor immunological microenvironment as a key mechanism in HCC (Fig. 2) [34,36,37,57-61].
A preclinical study exploring NRP1 and NRP2 in Mdr2 deficient HCC mice, found their expression higher in macrophages than in hepatocytes [57]. Additionally, thymocytes isolated from mice bearing a transplantable hepatoma 22a had higher NRP1 levels than control animals [58]. Among liver immune cells, dendritic cells expressed NRP1, correlating with RAD51 [34] and transcription factor activating enhancer binding protein 4 (TFAP4) [59], valuable prognosis and immune response markers, respectively, in patients with HCC [34,59].
Otherwise, several studies have described significant correlations between NRP1 and different immune response mediators in the HCC tumor microenvironment. One study found NRP1 was inversely correlated with interleukin-10 (IL-10) in patients with HCC [60]. However, another study found no significant correlation between NRP1 and killer cell lectin-like receptor B1 (KLRB1) [61]. Similarly, NRP1 downregulation via siRNA silencing increased interferon gamma (IFN-γ) [36] and IFN-β levels [37], and decreased tumor necrosis factor-α (TNF-α) levels [37] in two independent studies employing in vivo HCC models [36,37]. Therefore, while the exact mechanisms remain unclear, NRPs might exert an interesting function in the tumor-associated immune response in HCC.
NRPs and miRNAs
MiRNAs are highly conserved small noncoding RNAs that modulate gene transcription by binding to target messenger RNAs (mRNAs) [62,63]. They are differentially expressed among tissues and cancer stages, and are frequently dysregulated during oncogenesis [62]. The role of miRNAs in cancer development and progression gained attention given their broad underlying modulating processes [62,63]. In HCC, several miRNAs have been associated with tumor progression through their control of cell proliferation, invasion, migration and HCC development acting either as tumor suppressors or promoters [62,63]. Moreover, various miRNAs have shown to directly modulate target genes involved in critical tumor-associated processes [62].
Several studies have evaluated the interplay between miRNAs and NRP1, and the underlying mechanisms (Fig. 2) [40,51,64,65]. NRP1 has shown to be a target of miR-148b [40,51] and miR-340-5p/miR-452-5p [65], in both in vitro and in vivo HCC models. In addition, miR-124 or circular RNA (circRNA) circ-ABCB10 overexpression increased NRP1 levels in the HepG2 [64] and Hep3B [65] HCC cell lines, respectively. Interestingly, miR-148b-induced NRP1 downregulation strongly decrease cell migration in vitro [51], and tube formation and cell division in an HCC mouse model [40]. However, despite these novel findings, further studies should be performed to clarify the interplay between miRNAs and their associated effects in HCC.
Association between NRPs and the tumor microenvironment
The tumor microenvironment is a key factor in cancer development and progression that undergoes dynamic changes involving various components [66]. Cancer stem cells (CSCs), HSCs, cancer-associated fibroblasts (CAFs), cytokines and growth factors are tumor microenvironment components strongly associated with HCC progression [66-68]. Numerous studies have assessed the modulatory effects of the tumor microenvironment on NRP expression and activity, recently reporting some exciting findings (Fig. 2) [15,26,29,33,40,53].
CSCs are a small population of tumor cells that control differentiation, tumorigenicity, metastasis, and therapeutic resistance in HCC [67], with NRP1 appearing to play a key role [26,40]. Specifically, NRP1 downregulation in an in vitro HCC model significantly decreased the liver CSC population [26]. In contrast, NRP1 was overexpressed in the CSC population of two HCC cell lines [40] and expressed explicitly in CAFs and tumor-associated endothelial cells in HCC patients [33]. Among the cell types influencing the tumor microenvironment, HSCs have been broadly associated with HCC progression [66]. However, only one study evaluated NRP1 expression in HSCs, finding increased HepG2 cell proliferation in matrix conditioned with HSCs overexpressing NRP1 [29].
Low oxygen conditions, hypoxia, are crucial in HCC development, progression and chemoresistance [68-70]. A hypoxic microenvironment modulated both NRPs in vitro, decreasing NRP1 levels but increasing NRP2 levels (Fig. 2) [53]. Intriguingly, this study found that hypoxia decreased NRP expression when VEGF was silenced [53]. Similarly, cobalt chloride-induced hypoxia decreased NRP1 expression in the liver L02 cells [15]. Moreover, hypoxia directly modulated NRP1 in an in vivo HCC model, with NRP1 levels decreased in peritumoral tissue and negatively correlated with hypoxia-inducible factor 1-alpha (HIF-1α) levels [15]. Overall, both NRPs appear to contribute to tumor microenvironment modulation, showing key interactions with different tumor-associated cell types and the oxygen conditions in HCC.
DISCUSSION
While NRPs were described as key proteins in the nervous system through axon guidance modulation [10], recent findings also indicate an intriguing role in HCC and other cancer types [7,8].
Numerous studies have indicated a potential role for NRP1 as a tumor biomarker in HCC [12,14,16-20,23,24,26], while fewer and contradictory results have been reported for NRP2 [13,21,28]. Studies have found both NRPs overexpressed in tumor samples from different cancer types, including cholangiocarcinoma [18,71-75], supporting mainly NRP1, but also NRP2, as potential biomarkers in HCC. However, further studies are needed to clarify their exact role and improve the diagnostic tools available for human HCC. Moreover, both NRPs are strongly associated with worse prognoses and different tumor-associated parameters in HCC patients [15,16,24-26,29-33,35]. Similar findings were found with different solid tumors, where NRPs negatively correlated with prognosis and higher invasion and metastasis risk [18,71,73-75], highlighting their potential role as diagnostic tools for HCC patient prognosis.
Most studies evaluating NRP function in HCC have used preclinical models, where mainly NRP1, but also NRP2, exhibited an interesting modulatory roles by promoting cell survival, tumor progression, invasion and migration, and inhibiting apoptosis [12,15,17,26,35-48,51-54]. Similar findings were reported for other tumor types, describing TGF-β and other signaling pathways as potential targets of NRP regulation [76-79]. Additionally, invasion, migration and metastasis were also found to be strongly modulated by both NRPs in different solid tumor models [24,80,81], supporting roles for NRP1 and NRP2 in mechanisms underlying tumor progression and spread. Nevertheless, while these findings highlight interesting function for NRPs in HCC tumor progression and invasion abilities, further studies are needed to identify the exact mechanisms and signaling pathways modulated by NRP1 and NRP2.
Based on solid tumor heterogeneity and multiple processes involved in tumor cell adaptation and progression, numerous studies have described key functions for NRP1, and to a lesser extent NRP2, in modulating the immune response against tumor hepatocytes [34,36,37,57-61], that are associated with the potential interplay between NRPs, miRNAs [40,51,64,65], and the tumor microenvironment [15,26,29,33,40,53]. Other studies have also reported a correlation between NRP1 [82-85] and NRP2 [86] expression in different immune cells and immune response suppression in other cancers [82-86]. Specifically, NRPs had a crucial role in modulating the immune response [10,82], where NRP1 appeared to be associated with immune response suppression in cancer [82]. Regulatory T cells with depleted NRP1 exhibited decreased function, restoring the antitumor immunity and TNF-α production [83]. Moreover, NRP1 expression in macrophages, dendritic cells and other associated cell populations was associated with a restrained inflammatory response [84,85]. NRP2 expression in tumor-associated macrophages promoted tumor growth by regulating macrophage phagocytosis [86]. Therefore, based on these findings in different HCC models, both NRPs potentially play a key role in modulating the tumor-associated immune response, making them potential biomarkers in the HCC tumor landscape.
Consistent with growing evidence reinforcing the key role of miRNAs in cancer, these non-codifying RNAs modulate NRP1 in HCC, increasing tumor hepatocyte proliferation and migration [40,51,64,65]. Different miRNAs (e.g., miR-376a) or circRNAs (e.g., circ-LDLRAD3) regulated tumor progression in other cancer models [76,78]. While few studies have provided clearer results on the mechanisms underlying the potential interplay between miRNAs and NRPs, these findings suggest that miRNAs could be potential modulators of NRPs expression and activity, but mainly of NRP1, controlling key processes involved in HCC progression and invasion.
Several investigations have described that NRPs, primarily NRP1, are highly influenced by the tumor microenvironment, with tumor-associated cell populations playing a crucial role [87-89]. Among them, CAFs and CSCs could be modulated by NRP1 or NRP2 in different cancer types [87-89]. Indeed, NRP1 appears to have an interesting role in the response of different cell types in the tumor microenvironment, acting as a potential modulator of tumor adaptation and progression. Moreover, the interplay between hypoxia and NRPs has recently been explored in other cancers [90-92]. However, these results showed an opposite hypoxia effect to HCC, with increased NRP1 [91] but decreased NRP2 expression under hypoxic conditions [92]. Together with the studies on HCC, these results indicate that further investigations are needed to obtain a clearer understanding of the exact mechanism through which hypoxia and NRPs might contribute to tumor development.
Limitations
This review aimed to provide a clear and complete understanding of the main mechanisms modulated by NRPs in HCC development and progression. Nevertheless, some limitations exist that are mostly associated to the high heterogeneity among studies. The main limitation was that most articles examined only one NRP, with 40 articles focused on NRP1 but only four on NRP2; five examined both NRPs. This limitation led to greater discordance in the results obtained, mainly for NRP2, increasing the uncertainty of the conclusions drawn. Moreover, as shown in Table 1A, the methods employed for determining NRP1 or NRP2 levels were inconsistent, with most articles focusing on one NRP, using different targeting strategies. Discrepancies between studies could be explained by the different methods used to measure NRPs and the chosen targeting strategy.
Additionally, while multiple cellular and molecular processes had been evaluated, the number of studies analyzing each mechanism was highly heterogeneous. The diagnostic and prognostic values of NRP1 and NPR2, and their crucial role in invasion and migration, were the main processes studied, with few articles focusing on their interplay with miRNAs or the tumor microenvironment. While these interactions are key mechanisms in cancer development and progression, only five and six studies have explored them in HCC, respectively.
Finally, although increasing numbers of human studies have been published, they have not always described the main characteristics of HCC patients, with etiology, age, or country missing in some articles. Moreover, public databases hindered data extraction by not stating the number of patients included in the analysis. In summary, some important limitations should be considered when understanding and interpreting the main conclusions of this systematic review.
CONCLUSIONS AND FUTURE PERSPECTIVES
To the best of our knowledge, this article is the first systematic review focusing on the role of NRPs in HCC, summarizing all the results obtained from preclinical and clinical studies (Fig. 3). Increasing evidence suggests vital roles for these receptors (NRP1 and NRP2) in tumor-associated processes. The results summarized here suggest that NRP1 could act as a potential diagnostic biomarker and, with NRP2, an interesting prognostic biomarker in HCC patients. The NRPs have modulatory effects on different signaling pathways that promote tumor progression and are crucial mediators of the HCC cell invasion and migration abilities. The tumor-associated immune response is also strongly associated with NRPs, mainly NRP1, and the tumor microenvironment, in which different tumor cell populations have higher NRP1 levels. The interplay between miRNAs and NRPs has gained interest since several miRNAs directly modulate NPR1, restraining tumor cell proliferation. In summary, NRPs appear to have critical roles in various processes involved in tumor development and progression, suggesting the potential of both, but mainly NRP1, as tumor biomarkers and potential targets for improving the HCC patient outcomes.
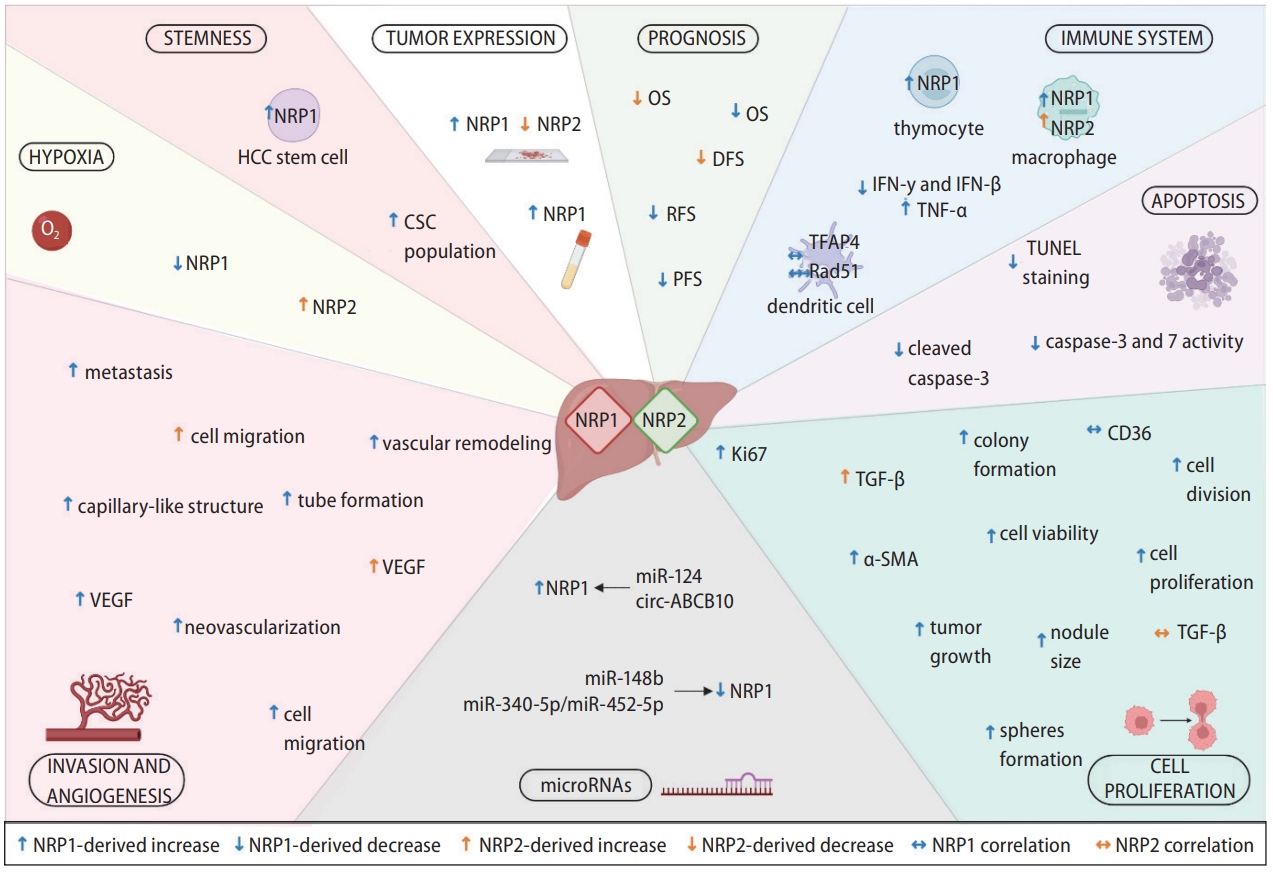
Main findings from the studies included in this systematic review describing modulatory effects associated to NRP1 and NRP2 in HCC. Specific modulatory effects exerted by both NRPs are graphically shown, together with correlations observed in different cellular processes and molecular mechanisms. α-SMA, α smooth muscle actin; CSC, cancer stem cell; DFS, disease-free survival; IFN-β, interferon beta; IFN-γ, interferon gamma; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival; TFAP4, transcription factor activating enhancer binding protein 4; TGF-β, transforming growth factor beta; TNF-α, tumoral necrosis factor-α; VEGF, vascular endothelial growth factor.
Notes
Authors’ contribution
All authors were responsible for study conception and design, interpretation of the data, and drafting of the manuscript. Systematic literature review, data extraction, and data analysis were performed by P.F.-P., T.P.-S., C.M.-B. and B.S.-M. In addition, M.J.T., J.G.-G. and J.L.M. supervised the study, and carried out the review and editing of the paper. The final version of the manuscript was approved by all authors.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This work was supported by the Ministry of Science and Innovation (MCIN/AEI/10.13039/501100011033) [project PID2020-119164RB-I00]. CIBERehd is funded by Instituto de Salud Carlos III (ISCIII), Spain. P.F.-P. is supported by the Ministry of Education (MCIN/AEI/10.13039/501100011033) [grant FPU17/01995] and T.P.-S. by the Asociación Española Contra el Cáncer (AECC)-Junta Provincial de León, Spain.
Supplementary materials
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
PRISMA 2020 checklist
PRISMA 2020 for abstracts checklist
Full search strategy employed for each online database (up to and including August 31st, 2022)
Evaluation of the published articles. (A) Temporal distribution of the number of articles published employing in vitro, in vivo or human models, or a combination of them. Comparison of the number of studies conducted with (B) human or (C) animal models with the mean of the human or animals examined per year, respectively.
Abbreviations
α-SMA
α smooth muscle actin
Alb
albumin
CAFs
cancer-associated fibroblasts
CD36
cluster of differentiation 36
CRC
colorectal cancer
CSCs
cancer stem cells
EMT
epithelial-to-mesenchymal transition
FasL
Fas ligand
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
HIF-1α
hypoxia-inducible factor 1-alpha
HSCs
hepatic stellate cells
IFN-γ
interferon gamma
IGFBP3
insulin-like growth factor binding protein-3
IL1R2
interleukin 1 receptor 2
IL-10
interleukin-10
KLRB1
killer cell lectin-like receptor B1
miR-148b
microRNA-148b
mRNA
messenger RNAs
NRP
neuropilin
OS
overall survival
PDGF-BB
platelet-derived growth factor-BB
PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses: PROSPERO
RFS
recurrence-free survival
SERPINA12
serpin family A member 12
TFAP4
transcription factor activating enhancer binding protein 4
TGF-β
transforming growth factor beta
TNF-α
tumor necrosis factor-α
VEGFA
vascular endothelial growth factor A
VEGFR3
VEGF receptor 3