The independent effect of exercise on biopsy-proven non-alcoholic fatty liver disease: A systematic review
Article information
Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease worldwide. Although previous studies have demonstrated that exercise independently reduces hepatic steatosis measured by imaging modalities in NAFLD, the effect of exercise on histological endpoints remains unclear. We aimed to conduct a systematic review of the independent effect of exercise on hepatic steatosis, steatohepatitis, and liver fibrosis as measured by histological assessment or non-invasive tests (NITs) in biopsy-proven NAFLD. A systematic literature search of PubMed, Embase, and Web of Science databases was performed using keywords related to exercise, NAFLD, and biopsy. Articles were selected based on the following inclusion criteria: (1) involved human subjects with biopsy-proven NAFLD, (2) analyzed the independent effect of exercise, (3) assessed changes in hepatic steatosis, steatohepatitis, or liver fibrosis via either histological evaluation or NITs, and (4) were original research studies. We identified a total of six studies that analyzed the independent effect of exercise on histological endpoints in biopsy-proven NAFLD. Two randomized controlled trials (RCTs) did not detect significant histological improvement following exercise interventions, while other non-randomized interventional studies showed that exercise reduces hepatocyte ballooning and liver fibrosis. In addition, five studies assessed NIT outcomes, collectively demonstrating that exercise improves hepatic steatosis measured by magnetic resonance imaging-based techniques but not serum biomarkers for steatohepatitis and liver fibrosis. Additional large RCTs and meta-analyses are warranted to investigate the independent effect of exercise on histological and clinical outcome endpoints in NAFLD.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD), recently redefined as metabolic-associated fatty liver disease (MAFLD) [1,2], has emerged as the most common etiology of chronic liver disease worldwide and is a leading cause of cirrhosis and hepatocellular carcinoma [3,4]. The global prevalence of NAFLD is projected to increase from 25% to over half of the adult population by the year 2040 [5,6]. NAFLD represents a spectrum of liver disease ranging from non-alcoholic fatty liver (NAFL) with bland steatosis to non-alcoholic steatohepatitis (NASH), a condition characterized by liver inflammation and hepatocellular damage that may cause progressive fibrosis leading to cirrhosis. Currently there is no approved pharmacological therapy for the treatment of NAFLD. As such, lifestyle modifications including exercise, diet, and weight reduction remain the cornerstone of NAFLD management [7,8].
An increasing number of randomized controlled trials (RCTs) and meta-analyses in the past decade have assessed the impact of exercise on NAFLD independent of other lifestyle interventions [9-14]. The vast majority of these studies, however, focus on the effect of exercise on imaging-based measures of hepatic steatosis. Given that only a few studies involve biopsy-proven NAFLD, limited evidence is available to address the impact of exercise on NASH resolution and liver fibrosis, the two primary regulatory endpoints for NASH drug development. Thus, we conducted a systematic review to (1) summarize the literature on the independent effect of exercise on hepatic steatosis, steatohepatitis, and liver fibrosis as measured by histological assessment or non-invasive tests (NITs) in biopsy-proven NAFLD, and (2) highlight the need for additional research centered on analyzing histological and clinical outcomes associated with exercise interventions.
METHODS
We conducted a systematic literature search using PubMed, Embase, and Web of Science databases from inception to October 10, 2022 to identify original research studies on the independent effect of exercise on hepatic steatosis, steatohepatitis, or liver fibrosis measured by histological assessment or NITs in human subjects with biopsy-proven NAFLD (Fig. 1). The search was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15] using the following keywords: (exercise, physical activity, physical endurance, physical exertion, physical training, endurance exercise, endurance training, aerobic exercise, aerobic training, walking, jogging, running, treadmill, swimming, resistance exercise, resistance training, progressive resistance, weight training, weight lifting, muscle exercise, muscle training, strength training, interval training, high-intensity interval, or HIIT) and (non-alcoholic fatty liver disease, fatty liver, hepatic steatosis, NAFLD, non-alcoholic steatohepatitis, steatohepatitis, or NASH) and (biopsy, histology, histologic, histological, histopathology, histopathologic, or histopathological).
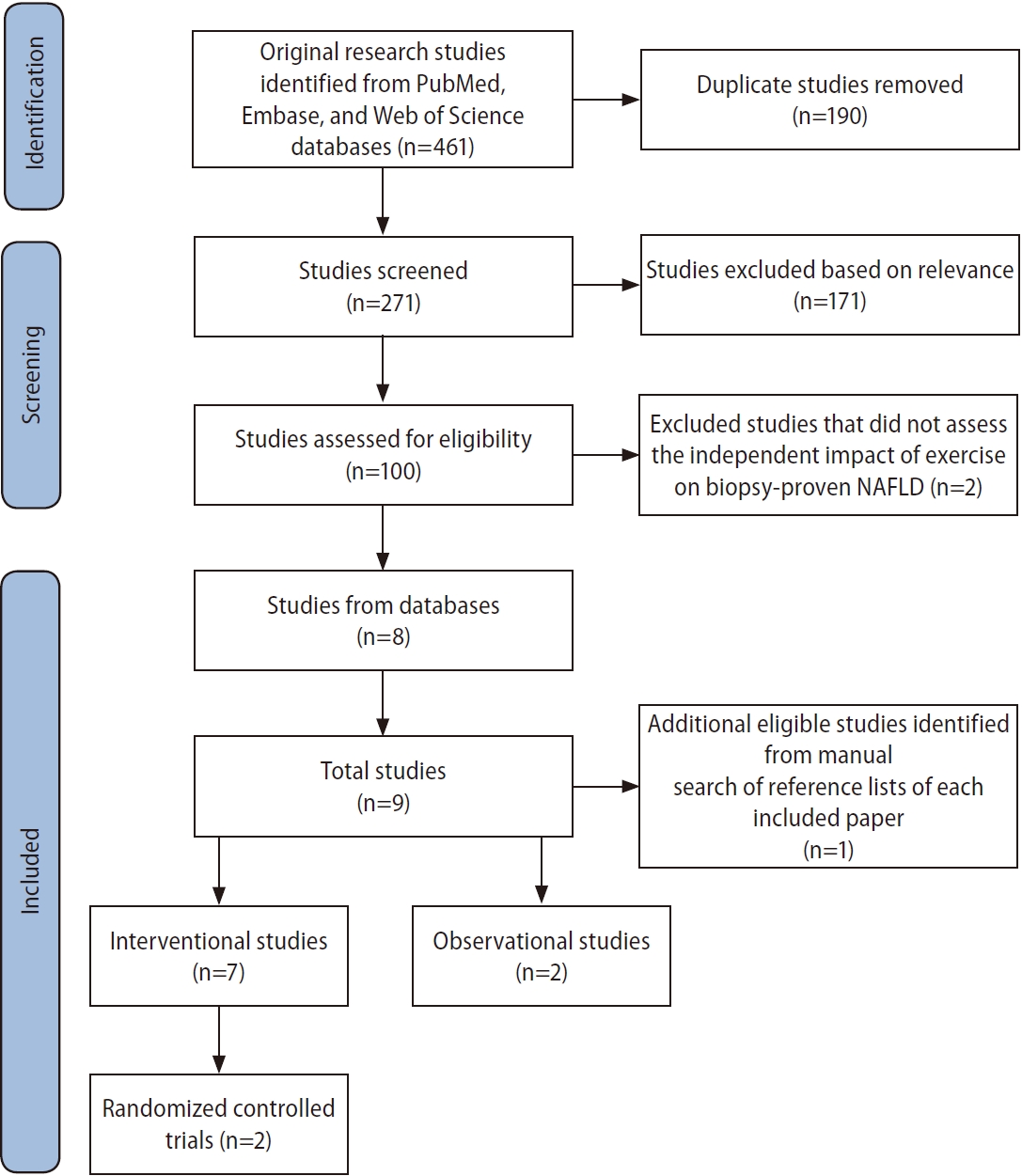
Identification, screening, and inclusion of studies for review. NAFLD, non-alcoholic fatty liver disease.
After removing duplicates, we included articles that met the following inclusion criteria: (1) involved subjects with biopsy-proven NAFLD, (2) analyzed the independent effect of exercise, (3) assessed changes in hepatic steatosis, steatohepatitis, or liver fibrosis via either histological evaluation or NITs, and (4) were primary research studies. Reference lists of each included paper were then manually reviewed to identify additional eligible studies.
RESULTS
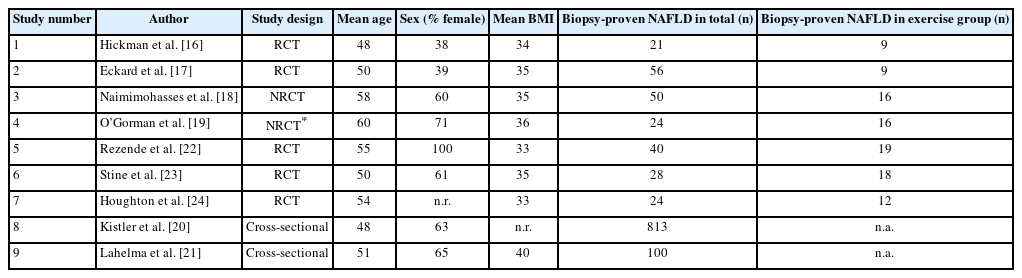
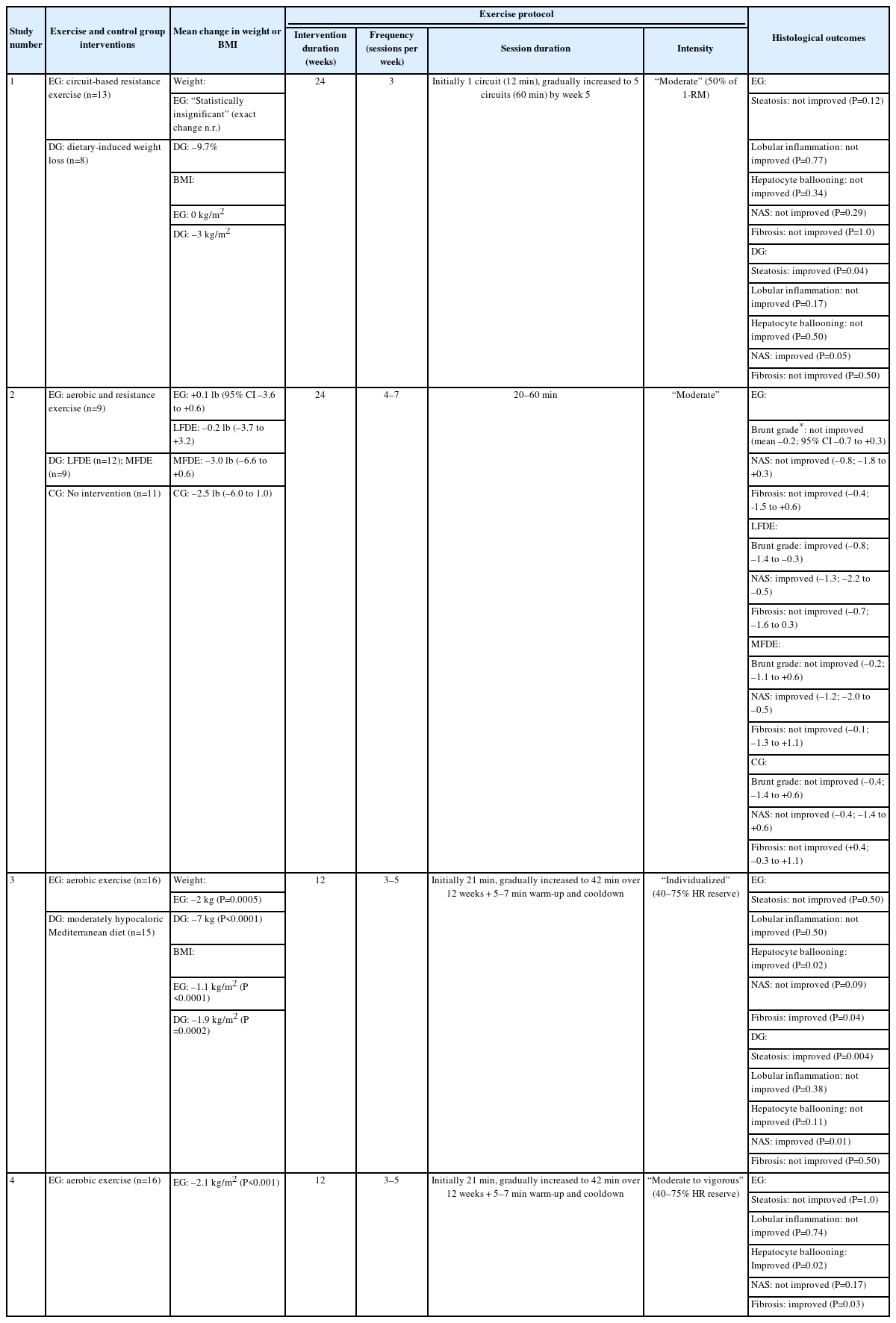
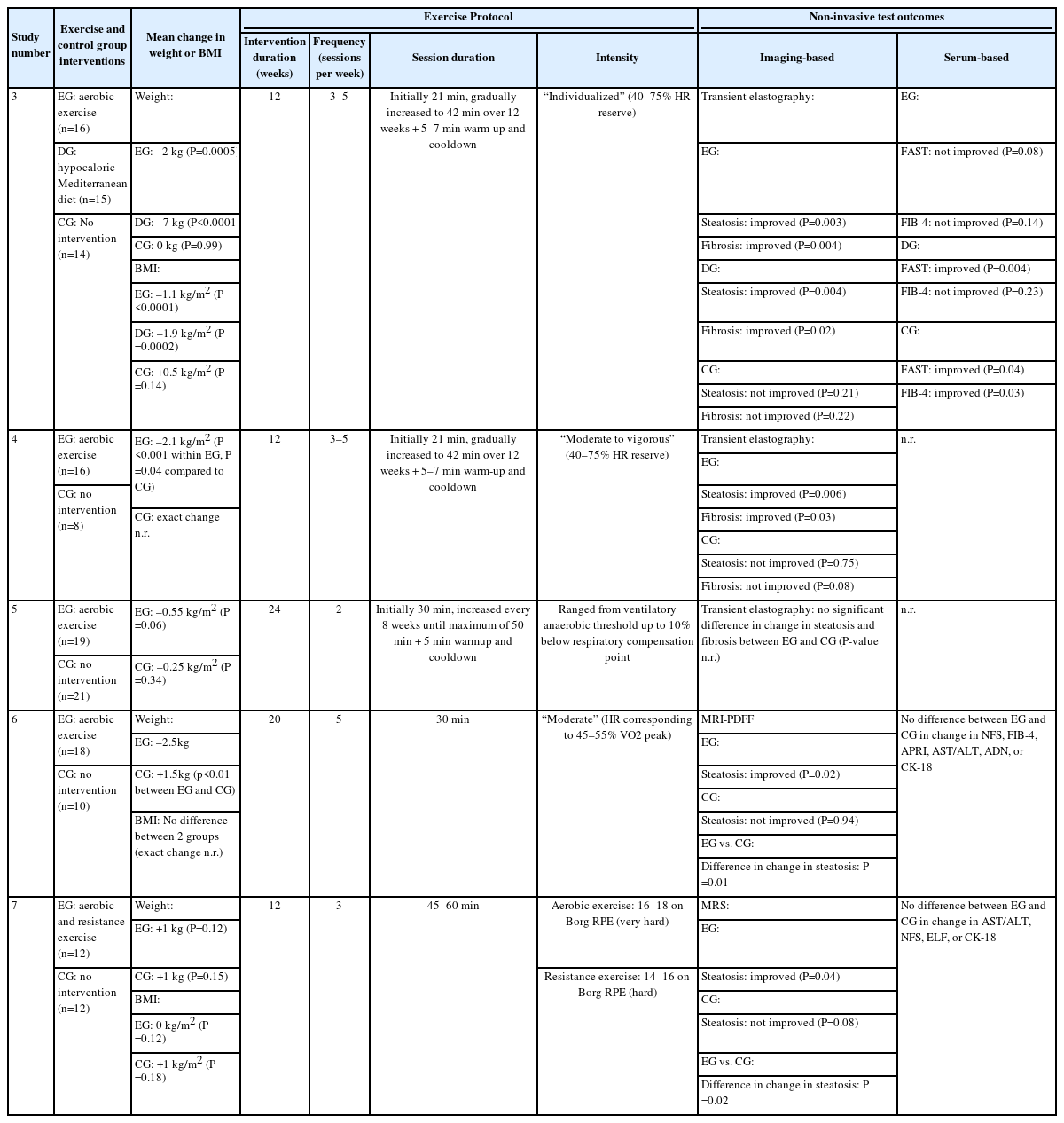
Our literature search yielded a total of nine studies, including seven interventional studies and two observational reports, that investigated the independent effect of exercise on hepatic steatosis, steatohepatitis, or liver fibrosis in biopsyproven NAFLD (Fig. 1). Six studies evaluated histological endpoints, and five studies assessed NIT outcomes [16-24]. The participant demographics of included studies are shown in Table 1. Protocols and results of interventional studies measuring histological and NIT endpoints are summarized in Tables 2 and 3.
Impact of exercise on biopsy-proven NAFLD assessed by histological evaluation
Two of the six studies that assessed histological endpoints were RCTs, neither of which reported statistically significant histological improvement following exercise interventions [16,17]. Hickman et al.16 randomly assigned 21 adults with NAFLD, 18 of whom had biopsy-proven NASH, to six months of either circuit-based resistance exercise without dietary changes or dietary-induced weight loss (DIWL). The exercise intervention consisted of three moderate-intensity sessions per week, starting with one circuit (12 minutes) per session during the first week and then a gradual increase to five circuits (60 minutes) per session by the fifth week. Supervision was offered to participants but not strictly required for the exercise intervention, resulting in an attendance rate of 90% for supervised sessions. The DIWL group achieved significant weight loss (mean –9.7%) while the exercise group did not. Post-intervention liver biopsies were performed in 14 participants (11 with NASH), revealing a significant decrease in both steatosis severity and NAFLD activity score (NAS) in the DIWL but not the exercise group. Neither group experienced significant change in lobular inflammation, hepatocyte ballooning, or fibrosis stage. Within the NASH-only cohort, two of the three participants in the DIWL group achieved NASH resolution while two of the eight participants in the exercise group achieved NASH resolution but this difference was not statistically significant (P=0.49) [16].
In another RCT, Eckard et al. [17] reported that a combination of aerobic exercise and resistance training did not result in significant histological improvement. Fifty-six subjects with NAFLD, including 36 with biopsy-proven NASH, underwent one of four interventions for six months: (1) low-fat diet plus exercise (LFDE), (2) moderate-fat/low-processed-carbohydrate diet plus exercise (MFDE), (3) exercise only, or (4) standard of care with basic nutrition and exercise education. Exercise intervention consisted of supervised moderate-intensity aerobic and resistance training sessions lasting 20–60 minutes each and occurring four to seven days per week. None of the four groups achieved significant weight loss following their interventions. While both the LFDE and MFDE cohorts experienced a significant decrease in NAS and the LFDE cohort achieved a significant improvement in Brunt grade, the exercise only group did not experience a significant change in either NAS or Brunt grade. None of the groups experienced significant change in fibrosis stage. Among the 36 participants with NASH, 19 (53%) saw an improvement in either Brunt grade or fibrosis including nine (25%) who had resolution of NASH. However, the authors did not report the distribution of patients with NASH across the four groups and did not distinguish NASH from NAFL as an endpoint, thereby preventing assessment of the independent impact of exercise on NASH. In addition, results for individual components of the Brunt grading system (steatosis, lobular inflammation, and hepatocyte ballooning) were not reported [17].
Two additional interventional studies evaluated the impact of exercise on histological endpoints in NAFLD [18,19]. Naimimohasses et al. [18] conducted a non-randomized controlled trial (NRCT) comparing exercise and diet interventions among 31 subjects. The exercise group participated in two supervised and one to three unsupervised aerobic exercise sessions per week, with each session lasting 21–42 minutes at 40–75% heart rate reserve, while the diet group followed a moderately hypocaloric Mediterranean diet. After 12 weeks of intervention, the exercise and diet groups experienced significant mean weight reductions of 2 kg and 7 kg, respectively. Upon histological evaluation, the exercise intervention elicited a significant improvement in both hepatocyte ballooning (P=0.02) and fibrosis (P=0.04) but not steatosis (P=0.50), lobular inflammation (P=0.50), or NAS (P=0.09). In contrast, the dietary intervention significantly reduced both steatosis and NAS but not fibrosis, hepatocyte ballooning, or lobular inflammation [18].
The exercise-induced histological changes reported by Naimimohasses et al. [18] were concordant with those found by O’Gorman et al. [19] in an uncontrolled interventional trial of similar study design. Sixteen participants with biopsy-proven NAFLD underwent a 12-week exercise intervention consisting of two supervised and one to three unsupervised moderateto-vigorous aerobic exercise sessions per week, with each session lasting 21–42 minutes at 40–75% heart rate reserve.
The exercise intervention led to significant reduction of body mass index (BMI), although none of the participants achieved the recommended ≥7% weight loss for improving histological outcomes in NAFLD [7]. Exercise significantly reduced hepatocyte ballooning (P=0.02) and liver fibrosis (P=0.03) but not steatosis (P=1.0), lobular inflammation (P=0.74), or NAS (P=0.17). Thirteen subjects in the exercise group had biopsy-proven NASH but the study did not report separate results for the NASH cohort or the number of subjects who experienced NASH resolution, and was limited by the lack of a control group [19].
Two observational studies evaluated the association between exercise intensity and liver fibrosis in biopsy-proven NAFLD [20,21]. In a retrospective cross-sectional study of 813 subjects with biopsy-confirmed NAFLD enrolled in the NASH Clinical Research Network, Kistler et al. [20] found that participants who engaged in ≥75 minutes of vigorous-intensity exercise (metabolic equivalent [MET] value≥6) had significantly decreased odds of having NASH (odds ratio [OR] 0.65; 95% confidence interval [CI] 0.43–0.98) and those who participated in ≥150 minutes of vigorous-intensity exercise had significantly decreased odds of having advanced fibrosis (OR 0.53; 95% CI 0.29–0.97) in multivariate logistic regression analysis adjusting for age, sex, BMI, education, income, and glucose. However, neither moderate-intensity exercise (MET value 3–5.9) nor total volume of exercise was significantly associated with NASH or degree of fibrosis [20]. In another cross-sectional study of 100 participants with biopsy-proven NAFLD, Lahelma et al. [21] demonstrated that increased amount of moderate-to-vigorous activity (MET value>3)—measured by a combination of accelerometer readings and self-report questionnaires—was independently associated with decreased risk of NAFLD fibrosis (OR 0.94; P=0.02) [21]. Of note, these studies were limited by cross-sectional study design and self-reported physical activity data potentially leading to misclassification bias [20,21].
In sum, a total of six studies have analyzed the independent effect of exercise on histological endpoints in biopsy-proven NAFLD, including two RCTs, one NRCT, one uncontrolled trial, and two cross-sectional reports. Notable heterogeneity existed between studies in exercise type, frequency, and duration as well as in supervision level and distinction of NASH from NAFL. Studies similar in design reported concordant histological changes: the two RCTs did not detect significant histological improvement after six-month exercise intervention, whereas reduction of hepatocyte ballooning and fibrosis was reported in the NRCT and uncontrolled trial, both of which implemented an aerobic exercise intervention with nearly identical duration, frequency, and intensity [16-21].
Impact of exercise on biopsy-proven NAFLD assessed by non-invasive tests
Since the advent of NITs for hepatic steatosis and fibrosis, three RCTs to date have studied exercise-induced changes in non-invasive biomarkers of hepatic steatosis, steatohepatitis, or liver fibrosis in biopsy-proven NAFLD [22-24]. In the first such study published, Rezende et al. used transient elastography as a NIT for liver steatosis and fibrosis. The authors randomly assigned 40 post-menopausal women to 24 weeks of either semiweekly supervised aerobic exercise sessions each lasting 30–50 minutes or no exercise. Neither group achieved a significant reduction in BMI. Aerobic exercise did not significantly improve hepatic steatosis or fibrosis score compared to the non-exercising control group. Of note, the frequency of exercise in this study design was lower compared to that of other study exercise protocols. In addition, steatosis severity was unable to be measured in 30% of study participants due to large body habitus. Nonetheless, this is the only RCT to use transient elastography to analyze the independent effect of exercise on biopsy-proven NAFLD [22].
In another RCT involving noninvasive biomarkers, Stine et al. [23] compared changes in both liver steatosis quantified by magnetic resonance imaging-proton density fat fraction (MRI-PDFF) and serum biomarkers for liver fibrosis and NASH between exercise and standard of care in 28 participants with biopsy-proven NASH. Exercise intervention consisted of 20 weeks of five 30-minute supervised moderate-intensity aerobic exercise sessions per week. Significantly greater weight loss was observed in the exercise group compared to control group, although there was no significant difference in change in BMI. Exercise significantly decreased MRI-PDFF compared to standard of care (P=0.01). Moreover, forty percent of exercise subjects achieved at least a 30% relative reduction in MRI-PDFF—a commonly cited threshold for surrogate histological response [25]—compared to 13% of control participants (P<0.01). Changes in serum markers for liver fibrosis and NASH, including NAFLD fibrosis score (NFS), fibrosis-4 (FIB-4) index, AST-to-platelet ratio, AST-to-ALT ratio, and exploratory biomarkers adiponectin and cytokeratin 18 (CK-18), were not significantly different between the exercise and control group [23].
Similarly, Houghton et al. [24] investigated the effect of exercise on both hepatic triglyceride content (HTGC) measured by magnetic resonance spectroscopy and serum biomarkers for liver fibrosis and NASH compared to standard of care in 24 participants with biopsy-confirmed NASH. The 12-week exercise intervention in this RCT consisted of a combination of supervised aerobic and resistance exercise three sessions per week, 45–60 minutes per session. Neither the exercise nor control group experienced significant change in weight or BMI. The exercise group achieved significant improvement in HTGC but not in AST-to-ALT ratio, NFS, enhanced liver fibrosis test, or CK-18 relative to the control group [24].
Two additional NRCTs have investigated the effect of exercise on biopsy-proven NAFLD measured by NITs [18,19]. In the same NRCT as described above, Naimimohasses et al. [18] reported significant improvements in hepatic steatosis and fibrosis scores measured by transient elastography in both the exercise and diet groups after 12 weeks, but not in a standard of care control group. When compared to dietary modification, the exercise intervention led to a greater reduction in both steatosis (13.8% vs. 12.5% reduction) and fibrosis (27.6% vs. 20.8% reduction), although the authors did not state if these differences were statistically significant. For other measured serum NITs, the exercise group did not experience significant change in either the Fibroscan-AST (FAST) score or FIB-4 index, while the diet group achieved a significant improvement in the FAST score but not in the FIB-4 index. Interestingly, the control group saw significant reduction in both the FAST score and FIB-4 index [18].
In the same study as described above, O’Gorman et al. [19] used transient elastography to measure serial hepatic steatosis and fibrosis scores in two non-randomized groups: (1) 16 participants with biopsy-proven NAFLD (13 with NASH) who underwent a 12-week aerobic exercise program, and (2) eight subjects with biopsy-proven NAFLD (six with NASH) who underwent standard of care. When compared to baseline measurements within the exercise group, both hepatic steatosis and fibrosis scores significantly improved one week following the completion of the exercise intervention, only steatosis score significantly improved three months following the intervention, and neither steatosis nor fibrosis score significantly improved 12 months following the intervention. The authors also assessed group-by-time interactions between the exercise and control groups and found that the change in steatosis was significantly greater in the exercise group at one week following the intervention but not at three or 12 months. No significant difference in the change in fibrosis was observed between the two groups at any of the measured timepoints. Although the exercise and control groups were non-randomized and results for NAFL and NASH were not reported separately, this is the only study to assess whether exercise leads to sustained improvement in steatosis and fibrosis months after the conclusion of an exercise intervention in participants with biopsy-proven NAFLD [19].
In summary, a total of five studies, including three RCTs and two NRCTs, have analyzed the independent effect of exercise on biopsy-proven NAFLD using NITs for hepatic steatosis, steatohepatitis, or liver fibrosis [18,19,22-24]. All but one study implemented aerobic exercise regimens, with duration of intervention ranging from 12 to 24 weeks [18,19,22,23]. Three studies relied on transient elastography and reported different effects of exercise on hepatic steatosis and fibrosis scores [18,19,22] while the remaining two studies used MRI-based modalities that detected significant improvement in hepatic steatosis following exercise interventions [23,24]. In addition, three studies assessed serum biomarkers and did not report significant exercise-induced changes, although these biomarkers served as secondary outcomes and therefore may have been underpowered [18,23,24].
DISCUSSION
To our knowledge, this is the first systematic review to examine the independent effect of exercise on hepatic steatosis, steatohepatitis, or liver fibrosis measured by histological assessment or NITs in patients with biopsy-proven NAFLD. Large well-powered studies investigating the impact of exercise on biopsy-proven NAFLD are limited in number. Perhaps most notably, there is no RCT data demonstrating that exercise independently improves NASH or NASH-related fibrosis assessed by histological evaluation, in contrast to the numerous RCTs and meta-analyses confirming a causal relationship between exercise and reduction of imaging-based measures of hepatic steatosis [9-14]. Although four studies suggest that exercise may improve specific histological features such as fibrosis and hepatocyte ballooning, these have important methodologic limitations such as self-reported physical activity data, lack of a control group, or non-randomized study design [18-21]. The two published RCTs reported that in the absence of weight loss or dietary modification, exercise failed to significantly improve histological markers of NAFLD. However, given the limited statistical power of these RCTs, an independent effect of exercise on biopsy-proven NAFLD cannot be excluded [16,17].
Exercise has been proposed to independently target key metabolic and inflammatory pathways implicated in the development and progression of NAFLD [26]. For example, exercise may reduce hepatic steatosis by upregulating peroxisome proliferator-activated receptors and adiponectin levels which in turn improves insulin resistance and lipolysis [27,28]. Previous studies have also demonstrated the inhibitory effect of exercise on inflammatory mediators, such as interleukin-1 beta and tumor necrosis factor-a, involved in the pathogenesis of hepatocellular injury and fibrosis [27,29]. Understanding whether exercise in the absence of other lifestyle modifications adequately achieves histological improvement not only holds important clinical implications in the current management of NAFLD but is also relevant for the interpretation of future clinical trials evaluating novel investigational therapies. Carefully designed and adequately powered RCTs are needed to address the independent effects of exercise form, duration, and intensity on histological endpoints.
The challenges of conducting trials involving histological evaluation should be acknowledged, especially with regards to limited study recruitment and loss of follow-up associated with serial liver biopsies. In cases where histological assessment is unfeasible, NITs for NAFLD may serve as alternative endpoints. To date, five studies including three RCTs have used imaging or serum NITs in biopsy-proven NAFLD. These studies demonstrated that while exercise significantly reduces MRI-quantified hepatic steatosis, its effect on steatosis and fibrosis estimated by transient elastography remains unclear [22-24]. The different findings between these two imaging techniques may be explained by greater accuracy of MRI-based modalities in detecting steatosis and fibrosis compared to transient elastography [30,31]. Serum markers of fibrosis and exploratory biomarkers for NASH were also studied as secondary outcomes in three studies, and were not found to be significantly improved by exercise [18,23,24]. As the prevalence of NITs for detecting liver fibrosis and diagnosing NASH increases in the clinical setting, future studies involving exercise interventions should too incorporate commonly used NITs to improve applicability of findings.
Although physical activity is associated with lower all-cause mortality in NAFLD and reduced risk of hepatocellular carcinoma in the general population [32,33], there are no studies published to date that have investigated the effect of exercise on key clinical endpoints in NASH, including progression to cirrhosis and liver-related mortality. The lack of literature on these endpoints is unsurprising as measuring these outcomes often require long-term follow-up potentially leading to high attrition rates. In addition, studies have not shown sustained benefits of exercise on hepatic steatosis and fibrosis in NAFLD following the completion of exercise interventions [19,34]. Given the difficulty of implementing strictly supervised exercise programs for a prolonged duration, establishing methods of transitioning exercise interventions to the community setting to promote long-term exercise adherence may benefit patients with NAFLD. Furthermore, exercise is also only one subset of physical activity, and other types of physical activity, known as non-exercise activity thermogenesis, may be considered as additional interventions.
We acknowledge several limitations of our systematic review, including the lack of meta-analysis and formal risk-of-bias assessments of eligible studies. In addition, exploring the relationship between exercise and other outcomes such as inflammatory markers, metabolic alterations, and cardiorespiratory fitness fell outside the scope of our review. Nonetheless, we demonstrated the need for larger interventional trials to investigate the independent effect of exercise on hepatic steatosis, steatohepatitis, and liver fibrosis as well as key clinical endpoints in biopsy-proven NAFLD.
Notes
Authors’ contribution
GC, BB, AD, and JL contributed to the conceptualization and methodology of the review. GC conducted the literature search, extracted and interpreted data, and drafted the manuscript. GC, BB, AD, and JL critically revised the manuscript. JL supervised the study. All authors approved the final version of the manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
The authors gratefully acknowledge Alyssa Grimshaw for her assistance with the literature search.
Abbreviations
NAFLD
non-alcoholic fatty liver disease
NITs
non-invasive tests
RCTs
randomized controlled trials
MAFLD
metabolic-associated fatty liver disease
NAFL
nonalcoholic fatty liver
NASH
non-alcoholic steatohepatitis
PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
DIWL
dietary-induced weight loss
NAS
NAFLD activity score
LFDE
low-fat diet plus exercise
MFDE
moderate-fat/low-processed-carbohydrate diet plus exercise
NRCT
non-randomized controlled trial
BMI
body mass index
MET
metabolic equivalent
OR
odds ratio
CI
confidence interval
MRI-PDFF
magnetic resonance imaging-proton density fat fraction
NFS
NAFLD fibrosis score
FIB-4
fibrosis-4
CK-18
cytokeratin 18
HTGC
hepatic triglyceride content
FAST
Fibroscan-AST