Surveillance of the progression and assessment of treatment endpoints for nonalcoholic steatohepatitis
Article information
Abstract
Nonalcoholic steatohepatitis (NASH) is an aggressive form of nonalcoholic fatty liver disease (NAFLD) characterized by steatosis-associated inflammation and liver injury. Without effective treatment or management, NASH can have life-threatening outcomes. Evaluation and identification of NASH patients at risk for adverse outcomes are therefore important. Key issues in screening NASH patients are the assessment of advanced fibrosis, differentiation of NASH from simple steatosis, and monitoring of dynamic changes during follow-up and treatment. Currently, NASH staging and evaluation of the effectiveness for drugs still rely on pathological diagnosis, despite sample error issues and the subjectivity associated with liver biopsy. Optimizing the pathological assessment of liver biopsy samples and developing noninvasive surrogate methods for accessible, accurate, and safe evaluation are therefore critical. Although noninvasive methods including elastography, serum soluble biomarkers, and combined models have been implemented in the last decade, noninvasive diagnostic measurements are not widely applied in clinical practice. More work remains to be done in establishing cost-effective strategies both for screening for at-risk NASH patients and identifying changes in disease severity. In this review, we summarize the current state of noninvasive methods for detecting steatosis, steatohepatitis, and fibrosis in patients with NASH, and discuss noninvasive assessments for screening at-risk patients with a focus on the characteristics that should be monitored at follow-up.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a heterogeneous and silently progressive disease that affects roughly one-third (32%) of the global population [1,2]. With an alarming increase in both worldwide prevalence and incidence, NAFLD has become one of the most common causes of chronic liver diseases in the majority of industrialized areas [3,4]. Compared with nonalcoholic fatty liver (NAFL), which is characterized by bland steatosis, nonalcoholic steatohepatitis (NASH) is a more progressive phenotype of NAFLD characterized by hepatocyte injury, inflammation, and scarring. It has been estimated that around 25% of NAFLD patients will develop NASH, and 20% of patients with NASH will develop cirrhosis and hepatocellular carcinoma (HCC) in 20 to 30 years from disease onset [5]. In the past decade, liver-specific and overall mortality rates of NASH have been increasing rapidly, especially in the patients with obesity, type 2 diabetes mellitus (T2DM), and metabolic syndrome [6]. Early identification and targeted treatment for NASH are urgently needed to improve patient outcomes.
Currently, diagnosis and evaluation of the severity of NASH is still based on liver biopsy-proven histopathological assessment and scoring, and is therefore reliant on invasive liver biopsy. The main scoring systems for NASH consider liver fibrosis, inflammation, and steatosis [7,8]. Although a number of noninvasive tests and predictive models have been developed to characterize fibrosis in NASH patients, their diagnostic performance and clinical application can be improved. Since there are still no NASH-specific drugs that have been approved by major drug administration agencies worldwide, lifestyle interventions including dietary changes and exercise, with the purpose of 10% weight loss, are the most effective approaches for the management of fibrotic NASH and underlying cardiometabolic comorbidities [9]. Liver biopsy, the current “gold” standard for the diagnosis of NASH, is essential for both patient enrollment and efficacy assessment of phase 2b trials of drugs currently under development in addition to all phase 3 trials [10,11].
Accurate evaluation of the severity of NASH and the risk of progression to liver cirrhosis and HCC is essential for screening at-risk NASH patients and determining treatment responses (including NASH remission and cirrhosis prevention) to novel NASH drugs in clinical trials. In the present review, we discuss approaches used for the surveillance of the progression of NASH and assessment of treatment endpoints.
RISK OF NASH PROGRESSION
Fibrosis
Liver fibrosis is recognized as a determinant of liver-related morbidity and mortality in patients with NAFLD/NASH [12]. Previous studies have shown that significant fibrosis (≥F2) and advanced fibrosis (≥F3) are independently associated with overall mortality, liver transplantation, and liver-specific mortality in patients with NAFLD [13]. In one study, patients with fibrotic NAFLD had a lower survival rate after liver transplantation than those with non-fibrotic NAFLD, regardless of the presence of NASH [14]. A recent meta-analysis demonstrated that the risk of liver-related mortality, all-cause mortality, and requirement for a liver transplant increased with poorer biopsy-confirmed fibrosis stage [15]. According to the Finnish population-based FINRISK and Health 2000 studies with a median follow-up of 12.1 years, the crude incidence of liverrelated outcomes in NAFLD was 0.97/1,000 person-years, and outcomes were associated with noninvasive fibrosis stage [16]. Moreover, HCC risk was highest with cirrhosis, followed by noncirrhotic fibrosis and comorbid T2DM in a biopsy-proven NAFLD cohort [17]. Correspondingly, NASH patients with compensated cirrhosis may have fewer liver-related complications if fibrosis regression is evident, which presents as a decrease in NAFLD fibrosis score (NFS), liver stiffness measurements, and hepatic collagen and alpha-smooth muscle actin expression [18]. In addition, most of novel drugs in phase 3 clinical trials targeting NASH also target fibrosis with stage ≥F2 to prevent fibrosis progression and liver-related events. Therefore, identifying NASH patients with significant fibrosis or advanced fibrosis can be used to identify populations at high risk for progression to liver cirrhosis and HCC [19].
Inflammation: a trigger of fibrosis and carcinogenesis
Patients with simple steatosis are often considered to have a similar life expectancy to that of the general population, while patients with NASH are generally considered to have a lower life expectancy. In the presence of chronic inflammation, adipose tissue releases free fatty acids and toxic lipids, followed by fat accumulation, lipotoxicity, oxidative stress, and mitochondrial dysfunction in hepatocytes [20], leading to liver fibrogenesis and carcinogenesis. It has been reported that up to one-third of NASH patients without effective intervention will develop advanced liver fibrosis or cirrhosis, and potentially HCC [21]. Although a previous study investigated the impact of fibrosis on the prognosis of NAFLD patients, persistent hepatocyte injury or chronic inflammation in the liver is one of the driving forces of disease progression and carcinogenesis [22]. A further study confirmed that fibrosis progression is faster in NASH than NAFL and that NASH patients are at higher risk for HCC than NAFL patients; NAFL patients progress one fibrosis stage per 14.3 years, while patients with NASH progress one fibrosis stage per 7.1 years [23].
Metabolic dysfunction: cause or consequence?
Obesity is the most common cause of metabolic dysfunction, and is considered related to the epidemic of NAFLD. Overall obesity increases de novo lipogenesis and decreases β oxidation of free fatty acids and very low-density lipoprotein secretion, resulting in hepatocyte lipidosis and lipotoxicity. However, it should be noted that a large proportion of patients with NAFLD are lean or non-obese based on body mass index [24,25]. Approximately 8–19% of Asians with a body mass index (BMI) less than 25 kg/m2 also have NAFLD [26], and the prevalence of NAFLD in non-obese subjects has been found to be as high as 16% [24]. However, obesity as defined by BMI is only a crude measurement of obese status. Other anthropometric parameters might be useful for diagnosis of central obesity, occult obesity, and sarcopenic obesity. Central adiposity, sarcopenia, dyslipidemia, and insulin resistance are strongly associated with NASH and related fibrosis in a dose-dependent manner [27]. The progressive course of NASH is closely linked to an increasing number of metabolic comorbidities. T2DM has the strongest association with incident HCC in patients with NAFLD [28-30]. Metabolic syndrome is an independent predictor of all-cause, liver-specific, and cardiovascular mortality in patients with NAFLD [31,32]. In contrast, mortality of metabolically normal NAFLD patients is similar to that of patients without liver disease [33-35]. Thus, assessing metabolic dysfunction, including insulin resistance, may help define high-risk NASH patients [36]. In addition, accumulating evidence suggests that NAFLD has complex links with metabolic dysfunction; for example, NAFLD, especially NASH, is also associated with an increased risk of incident T2DM and atherosclerotic cardiovascular disease events [37].
HISTOPATHOLOGICAL SURVEILLANCE FOR NASH
Liver biopsy is imperfect
Screening of high-risk patients and surveillance for the development of liver-related complications are urgently needed for the management of NASH given the chronic progressive nature of this disease. Several novel NASH pharmacological agents are currently under development, and monitoring the treatment response relies on accurate assessment in clinical trials. Histopathological assessment is considered the “gold” standard for the diagnosis and evaluating of NASH severity and fibrosis stage. However, liver biopsy is not feasible for repeated assessment due to its invasive nature. Furthermore, histological evidence from liver biopsies is only moderately accurate and requires additional validation, therefore more reliable techniques for accurate quantification of the severity of NASH and fibrotic stage are required.
Histological classification of NASH is currently performed using semiquantitative scoring systems. NAFLD activity score (NAS) which was developed by the NASH clinical research network, and the steatosis, activity, fibrosis scoring system developed by fatty liver inhibition of progression Pathology Consortium, are the two most widely used scoring systems [7,38]. Both systems identify the location and features of fibrosis, number of inflammatory foci, number of balloon cells, and percentage of parenchymal involvement of the steatosis. Assessment depends on manual and subjective judgment, resulting in intra- and inter-observer variability. Although liver biopsy is generally considered safe and is widely available, histological scoring is limited by sampling error and ordinal classification. Developing innovative methods based on machine learning (ML), artificial intelligence (AI), and whole-slide images (WSI) may be a key to improve histopathological assessment.
Novel liver biopsy-based assessment tools
Second-harmonic generation (SHG) microscopy is highly sensitive to the collagen fibril/fiber structure, and has enabled the imaging of fibrillar collagen in various tissues. SHG-based novel technology has also been applied to assess hepatic fibrosis in chronic liver diseases [39]. HistoIndex as one of the SHG-based novel technologies for the assessment of hepatic steatosis has shown a good correlation with histopathologist scores [40], and was applied in a phase 2 clinical trial (MGL-3196, Resmetirom) to evaluate dynamic changes in steatosis during treatment [41]. A model to quantify fibrosis-related parameters (q-FPs) was developed by Wang et al. to assess the characteristics of liver fibrosis in NAFLD. A model containing four q-FPs (number of collagen strands, strand length, strand eccentricity, and strand solidity) was established based on findings in 50 test subjects and validated in 42 validation subjects to facilitate continuous and quantitative evaluation of fibrosis [42]. Furthermore, a combination of qFibrosis, qInflammation, qBallooning, and qSteatosis (qFIBS index) was developed to allow quantitative assessment of the characteristics of NAS (lobular inflammation, ballooning, and steatosis) by using SHG and two-photon excitation fluorescence imaging technology. qFIBS was developed and then validated in a cohort of 219 patients with biopsy-proven NAFLD/NASH and showed a robust correlation with NAS and fibrosis stages [43]. Recently, qFIBS was applied in a phase 2 trial of tropifexor (NCT02855164), to assess the resolution of NASH and fibrosis. qFIBS was found to have sufficient sensitivity to evaluate regressive changes in septa morphology and a reduction in septa parameters in F3 patients, which cannot be captured by traditional scoring systems [44].
Advances in machine-learning-based approaches are enabling histopathological monitoring of the progression and regression of NASH [45]. Digital WSI comprises scanning of hematoxylin-eosin -stained slides to quantify steatosis by assessing the steatosis proportionate area. Elastica van Gieson-stained slides can be scanned to quantify fibrosis by assessing the number of collagen and elastin fibers [46-48], and is regarded as an automated, precise, objective and quantitative method to assess NASH. Assessment of ballooning cells, one of the most important features of NASH, is highly subjective. AI-based technology can be trained to reproducibly quantify ballooned hepatocytes and standardize the evaluation [49]. ML-based models have been used to assess NASH histological characteristics accurately in addition to treatment response. PathAI showed concordance with ordinal grades from pathologists in terms of three NAS components. In addition, PathAI detected improvements in the DELTA Liver Fibrosis score in fibrosis responders in the combination group (cilofexor+firsocostat) in the ATLAS study [50]. AI- and ML-based technologies are advancing rapidly and can potentially address the inadequacies of pathological assessment of fibrotic NASH.
NONINVASIVE MARKERS ARE MORE PRACTICAL THAN LIVER BIOPSY FOR MONITORING OF NASH
Given the increasing prevalence of NASH, the base of at-risk patients who need screening is large. Liver biopsy is a critical bottleneck in the diagnosis and monitoring of these patients. Thus, it is critical to develop accurate noninvasive tests, markers, and models to evaluate NASH severity and monitor drug efficacy. Based on these needs, researchers have developed several noninvasive assessment methods including serum biomarkers, elastography-based markers, imaging studies, genetic tests, and omics profiling.
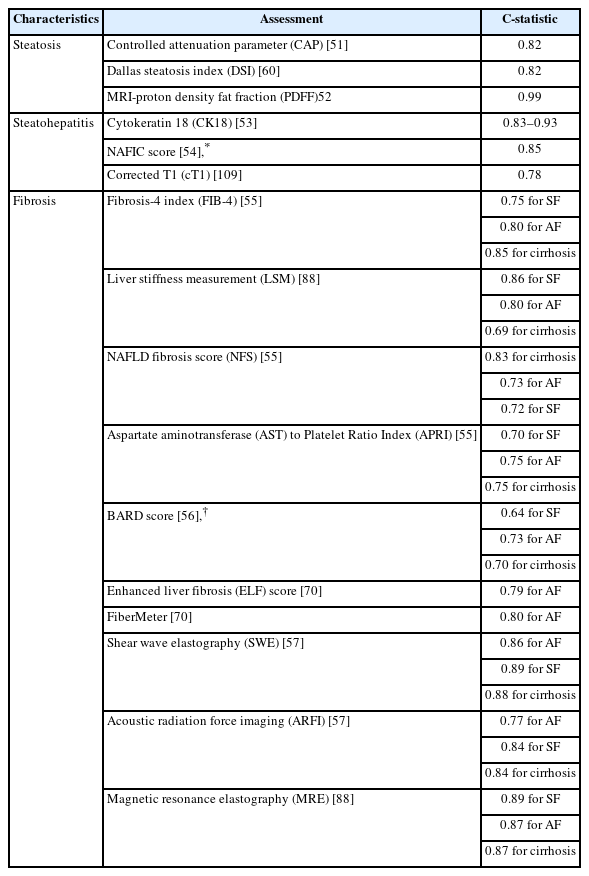
Noninvasive tests are more acceptable for evaluation of steatosis degree and fibrosis stage than liver biopsy, and also improve screening compliance and monitoring of NAFLD. As histological assessment from liver biopsy is still imperfect, an ideal solution is to link clinical outcomes such as cirrhosis, HCC, and liver-related complications with novel noninvasive markers. Correlating the histological severity of NASH and fibrosis stages with quantified noninvasive markers is a feasible approach (Table 1) [51-57].
Serum biomarkers for assessment of steatosis
Currently, the most promising noninvasive diagnostic tools for hepatic steatosis are the fatty liver index (FLI), the hepatic steatosis index (HSI), the NAFLD-liver fat score, the visceral adiposity index, the lipid accumulation product (LAP), and the triglyceride×glucose index [58]. Most of these indexes have been validated in biopsy-proven cohorts or magnetic resonance spectroscopy (MRS) results have been used as a reference. The accuracy of FLI, HSI, LAP, and the Zhejiang University index (ZJU) was evaluated in a general population by ultrasonography. Although FLI showed the highest C-statistic (0.85), the relatively low sensitivity of ultrasonography in detecting mild steatosis is of concern [59]. Although assessing steatosis grade is simpler than assessing inflammation or fibrosis, detecting >5% hepatic steatosis by circulating biomarkers alone is insufficient. Combinations of biomarkers would likely increase the accuracy of detecting steatosis. Dallas Steatosis Index (DSI), which consists of age, sex, diabetes, hypertension, race, BMI, serum triglycerides and alanine amiotransferase, was developed in the Dallas Heart Study of 737 patients with MRS-diagnosed liver fat. The C-statistic of DSI was found to be 0.82, but its diagnostic performance still needs external validation [60]. It should be noted that ultrasound tests are more widely available than blood-based tests. Serum proteins measured in these models are associated with metabolic disorders or insulin resistance and are not strictly specific to hepatic fat content, which may explain why these models have insufficient accuracy, especially in non-obese or lean subjects. The current serum-based noninvasive markers therefore have limited utility for surveillance.
Serum biomarkers for the assessment of liver fibrosis
Given that fibrosis is the major driver of liver-related outcomes in NAFLD, assessing fibrosis stage is essential for screening at-risk patients. The simple serum biomarker panel used in the fibrosis-4 index (FIB-4) and the aspartate aminotransferase (AST) to Platelet Ratio Index (APRI), originally developed for chronic viral hepatitis, could be applied in NASH patients. The cut-off value for FIB-4 is 2.67 and 1.30 to rule in and rule out advanced fibrosis in patients with NAFLD, respectively. NFS, developed from a liver biopsy-proven NAFLD cohort, has cut-off values of -1.455 and 0.676 to rule out or rule in advanced fibrosis [61]. BARD score (a scoring system include body mass index, AST/ALT ratio, and diabetes) was developed to diagnose advanced fibrosis by combining BMI, AST/ALT levels, and diabetic status [62]. Both FIB-4 and NFS are relatively easy to perform and are recommended for identification of NAFLD patients at low or high risk of advanced fibrosis. These tests have been widely used, and are available in primary health care units. However, due to the various etiologies of the cohorts who these makers were validated in, the accuracy of these tests needed to be improved when applied to NAFLD cohorts. In addition, models developed from biopsy-proven NAFLD cohorts often use higher cut-off values than those used for the general population. This leads to inferior diagnostic performance of NFS, FIB-4, and APRI in general population [63,64].
Many biomarker tests, including those with patented markers, involve direct biomarkers of fibrogenesis or fibrinolysis from the extracellular matrix. Type III collagen and hyaluronic acid (HA) are common biomarkers. The amino-terminal propeptide of type III procollagen (PIIINP) can discriminate between regular and advanced fibrosis with a C-statistic of 0.82-0.84 [65]. Enhanced liver fibrosis (ELF) test, a commercial panel of markers comprising serum HA, the PIIINP, and the tissue inhibitor of metalloproteinase-1 (TIMP-1), was first developed in children with NAFLD and validated in larger cohorts [66,67]. Recently, the ELF test was used to assess fibrosis improvement during aldafermin (NGM282) treatment [68]. Another type III collagen-based fibrosis algorithm including age, presence of diabetes, PRO-C3 (a marker of type III collagen formation), and platelet count (called ADAPT) showed better diagnostic performance than APRI, FIB-4 and NFS in predicting advanced fibrosis [69]. FibroMeter consists of age, weight, glucose, AST, ALT, ferritin, and platelets, and has been directly compared with ELF. ELF and FibroMeter had significantly higher C-statistics than NFS and FIB-4 in diagnosing advanced fibrosis, while the C-statistic did not differ significantly between ELF and FibroMeter [70]. FibroTest is a commercial panel with a C-statistic of 0.75–0.86 for significant fibrosis and 0.81–0.92 for advanced fibrosis [71]. FIBROSpect, which comprises alpha 2 macroglobulin, HA, and TIMP-1, is highly sensitive for advanced fibrosis (positive predictive value, PPV 92.5–94.7%), with a C-statistic of 0.86 [72]. Hepamet was developed in 2,452 biopsy-proven NAFLD patients, and had a higher C-statistic than FIB-4 and NFS. Hepamet is unaffected by age, BMI or diabetes [73]. These tests, although more accurate at predicting advanced fibrosis, are expensive, and there is still a dearth of direct comparisons in the same cohorts. In general, biomarkers or models detecting advanced fibrosis have a relatively high negative predictive value (NPV) while the positive predictive value (PPV) requires improvement.
Serum biomarkers to assess steatohepatitis
Hepatocyte ballooning and inflammation are the most important features of steatohepatitis, but current biochemical and imaging measures cannot effectively distinguish NASH from NAFL. Serum ALT is not a sufficiently sensitive predictive marker for diagnosis of steatohepatitis as less than 30% of NASH patients have elevated ALT levels (>35 U/L). Use of ALT >2 times the upper limit of normal to diagnose NASH only has 50% sensitivity and 61% specificity [74]. Cytokeratin 18 (CK18) is released into the serum on initiation of apoptosis in the form of CK18-M30 and CK18-M65 fragments. Serum CK18 has been the most widely investigated in the diagnosis of NASH. In one study, CK18 was thought to have potential predictive value for fibrosis, but showed a better correlation with ALT rather than with steatosis or fibrosis [75]. Another study involving repeated liver biopsy found that serum CK18 level was associated with NAS ≥5 (definite NASH) in patients with NAFLD [76]. Meta-analyses have confirmed that CK18 can predict steatohepatitis with a C-statistic around CK18 0.80 and sensitivity of 66–78% [77,78]. Index of NASH, which consists of waist-to-hip ratio, triglyceride, ALT, homeostatic model assessment for insulin resistance (HOMA) and gender, was developed to diagnose steatosis [79] but showed low sensitivity in an external cohort, especially in non-obese subjects [80]. Although serum level of hypersensitive C-reactive-protein (hsCRP) is included in the diagnosis of metabolic-dysfunction associated fatty liver disease [81] its diagnostic value in NASH requires further investigation. A recent study of 100 subjects observed an independent relationship between hs-CRP and NAFLD [82]. More direct evidence is required for use of hs-CRP as a diagnostic marker for NASH. Both single nucleotide polymorphisms and noncoding RNAs have been used to predict NASH. NASH Score (PNPLA3 genotype, AST, and fasting insulin) and circulating miR-122 have shown potential prognostic significance in NASH [83,84]. Unlike NASH-related fibrosis, there are currently no direct biomarkers for steatohepatitis. The available evidence indicates that use of a single biomarker to discriminate bland steatosis from NASH is unlikely to be successful.
Advances in imaging-based approaches
Ultrasonography is the most widely used imaging tool for identifying liver disease but lacks sensitivity. In patients with mild to moderate steatosis, the accuracy of ultrasonography is only around 50% [85]. Thus, quantitative ultrasound-based techniques are being developed to improve the diagnosis of hepatic steatosis. Attenuation coefficient (AC) and back scatter coefficient (BSC) have been shown to be correlated with the severity of hepatic steatosis. In a biopsy-proven study, AC and BSC achieved an accuracy of 61.7% and 68.3% in predicting steatosis grade, respectively, which are significantly higher accuracies that achieved with traditional ultrasonography [85]. Ultrasound-guided attenuation parameter has also showed excellent ability to distinguish steatosis grades (0.92, 95% confidence interval: 0.87–0.97) in non-B non-C chronic hepatitis subjects [86]. Transient elastography (TE) devices can be used to assess the controlled attenuation parameter (CAP) for liver fat quantification. CAP showed good sensitivity for detecting mild steatosis (S1) and excellent diagnostic accuracy in distinguishing S1, S2, and S3 in a study that used liver biopsy as the reference [87,88]. In terms of incidence and resolution of steatosis, CAP can also be used to assess dynamic changes [89]. Although the sampling error of CAP can be reduced by increasing the detection volume (3 cm3), its accuracy is reduced by increasing amounts of subcutaneous adipose.
Among magnetic resonance imaging (MRI)-based biomarkers, MRS is sensitive to small amount of hepatic adipose and is recognized as the most accurate noninvasive method to quantify steatosis. MRS is often used as the reference when assessing other noninvasive markers [59]. However, advanced training is required to measure MRS, which has limited its widespread application. MRI-proton density fat fraction (PDFF) is more accessible than MRS in most tertiary health centers. MRI-PDFF can assess the fat content in the whole liver and also allow for the assessment of regions of interest. Multiple studies have proven a close agreement between fat content as assessed by MRI-PDFF and histological steatosis grade [90,91]. Liver fat content measured by MRS or MRI-PDFF changes over time, which could reflect dynamic changes in hepatic steatosis. MRI-PDFF can be used to determine absolute and relative liver fat content. MRI-PDFF was shown to have better diagnostic accuracy than CAP in a head-to-head comparison [88].
Computed tomography (CT) can be used to assess liver fat content through the absolute attenuation of liver parenchyma value [92]. CT is more sensitive to moderate-to-severe steatosis than mild steatosis. The sensitivity for detecting grade ≥2 steatosis is more than 90%. Although CT is not routinely used to identify steatosis, it can be important in detecting incidental steatosis.
TE is the simplest and most commonly used noninvasive imaging tool for screening for fibrosis in clinics. The cut-off values of liver stiffness measurement (LSM) by TE for identifying advanced fibrosis varies with liver disease etiology. For NAFLD, a recent study determined a cut-off of 6.5 kPa to rule out advanced fibrosis and a cut-off of 12.1 kPa to rule in advanced fibrosis [93]. In a study of Asian NAFLD patients, the cut-off value to rule out advanced fibrosis was 7.9 kPa and the cut-off to rule in advanced fibrosis was 9.6 kPa [94]. LSM is sensitive to advanced fibrosis and cirrhosis, while its specificity for ruling out F1 and F2 fibrosis requires improvement. In addition, LSM can be affected by various factors including obesity, subcutaneous fat thickness, high ALT levels, and cholestasis [95]. Agile 3+ and Agile 4 are models that combine LSM with routine clinical parameters to identify advanced fibrosis and cirrhosis, respectively. Both Agile 4 and Agile 3+ showed better diagnostic performance, especially positive predictive value, than FIB-4 and LSM [96]. Acoustic radiation force imaging (ARFI) was developed from a chronic hepatitis C patient cohort to diagnose advanced fibrosis. The efficacy of ARFI, supersonic shear imaging (SSI), and TE was compared in a head-to-head study. Similar to TE, the application of ARFI and SSI in obese subjects is limited, and SSI showed higher accuracy than ARFI for diagnoses of F2 fibrosis [97].
MRI machines can be equipped with magnetic resonance elastography (MRE) to assess liver stiffness. Both MRE and TE showed excellent diagnostic accuracy for diagnosing stage F2-F4 fibrosis with a C-statistic of greater than 0.90 [98]. Several studies have reported that MRE is more accurate than TE [88,98,99]. MRE also has a higher success rate than TE at detecting fibrosis in obese patients (95.8% vs. 88.5%). In a recent meta-analysis, MRE had a higher C-statistic for detecting F≥2 and F≥3 but a similar performance to TE and shear wave elastography at detecting cirrhosis [100]. The combination of MRI with other imaging tests and biomarkers could increase diagnostic performance. MEFIB is the combination of MRE and FIB-4, and showed a relatively high PPV of 97.1% in diagnosing ≥stage F2 fibrosis [101]. The MRI-aspartate aminotransferase (MAST) score refers to the combination of MRI and NFS, FIB4, and FibroScan-aspartate aminotransferase (FAST). MAST had a higher C-statistic than that of the components of this index, reducing the number of the patients in the “gray zone” [102].
DYNAMIC MONITORING AND PROGNOSIS RISK ASSESSMENT
Definition and biomarkers of at-risk NASH patients
Given the progressive nature of NASH, there are numerous efforts underway to develop novel drugs. Emerging treatments mostly target hepatic fibrosis and steatohepatitis-associated inflammatory activity. Patients who are at risk of disease progression should therefore be included in clinical trials and effective tests should be used to repeatedly assess the drug response. The Liver Forum defined the following NAFLD subgroups: NAFL, indeterminate NASH, NASH without fibrosis, NASH with early fibrosis, NASH with bridging fibrosis, compensated cirrhosis, and decompensated cirrhosis [103]. A number of biopsy-proven studies have showed that both fibrosis stage and NAS at baseline are correlated with a higher risk of increased fibrosis stage during follow-up. Recently, Harrison et al. [104] defined “at-risk NASH” patients as NAFLD patients with NAS ≥4 and fibrosis stage ≥2. Following this definition, several studies have offered noninvasive solutions to distinguish these patients from others.
MACK-3 is the combination of AST, HOMA, and CK18, and has shown high accuracy in at-risk NASH patients (NAS ≥4 and F ≥2) [105]. Cut-off MACK-3 values of ≤0.134 and ≥0.550 can be used to rule out and rule in these patients who need more aggressive drug intervention, respectively [106]. The algorithm ADAPT mentioned previously is also effective at detecting at-risk patients [107]. A recent study compared the diagnostic performance of MEFIB, MAST, and FAST at detecting at-risk NASH patients. All three models provided utility in NAFLD risk stratification, while MEFIB showed better performance at detecting at-risk NASH than MAST and FAST [108]. Direct correlation with the severity of inflammation was previously regarded as the bottleneck of imaging tests, but currently corrected T1 (cT1) showed potential in predicting NASH. cT1 had better diagnostic accuracy (0.78 vs. 0.69) in identifying high-risk NASH than MRI-PDFF [109]. Furthermore, a protein-based signature of fibrosis could also serve as a diagnostic tool. A disintegrin, a metalloproteinase with thrombospondin motif like 2 (ADAMTSL2), and an 8-protein panel showed predictive value for at-risk NASH [110].
Biomarkers of treatment response and clinical outcomes
The best clinical outcome to evaluate the efficacy of NASH treatment is liver-related morbidity and mortality, while the surrogate endpoint is histologic outcome. Current guidelines recommend histological NASH resolution without worsening of fibrosis or regression of fibrosis without worsening of NASH as the treatment endpoint in phase 3 trials of NASH [11]. The reliance on histologic outcomes for primary trial endpoints is a barrier to patient enrollment. There is an urgent need to develop accurate noninvasive markers that reflect drug-induced changes. Markers or algorithms that reflect disease severity or long-term prognosis could be utilized as surrogate endpoints for clinical trials of drugs targeting NASH (Fig. 1).

Evaluation approaches for different trial phases and different stages of NASH. Specific sets of evaluation tools should be used for different phases of NASH. Different assessments are also required for patients with different stages of NASH. MRE, magnetic resonance elastography; MRI-PDFF, magnetic resonance imaging-proton density fat fraction; NASH, nonalcoholic steatohepatitis; NAS, NASH activity score; AEs, adverse events; CAP, controlled attenuation parameter; US, ultrasound; CK18, Cytokeratin 18; cT1, corrected T1; FLI, fatty liver index; HSI, hepatic steatosis index; FIB-4, fibrosis-4 index; LAP, lipid accumulation product; NFS, NAFLD fibrosis score; ELF test, enhanced liver fibrosis test; LSM, liver stiffness measurement; MELD score, model for end-stage liver disease score; HVPG, hepatic venous pressure gradient.
Some noninvasive markers reflect dynamic changes associated with histological changes. Imaging-based tests have the best potential to be surrogates of histological assessment of steatosis grade and fibrosis stage [111]. As early as in the FLINT trial of obeticholic acid (OCA), MRI-PDFF was used as a surrogate marker of steatosis. Taking a 30% relative reduction in MRI-PDFF as an endpoint, OCA was better than the placebo in achieving the goal. In addition, non-responders also showed less histological improvement than responders (19% vs. 50%, respectively) [112]. Patented ELF and PIIINP were also used as serum markers of treatment efficacy in the PIVENS Trial. ELF showed a significant correlation with advanced fibrosis in patients with NASH, but not with longitudinal changes in fibrosis [113]. As mentioned above, ML-based methods can be used to translate histological characteristics into continuous variables. For instance, collagen proportionate area (CPA) as assessed by digital image analysis may offer a more granular assessment of fibrosis than routine histological analysis. Small changes detected by CPA might be missed when comparing fibrosis stages [114,115]. Furthermore, ML-based histological assessment is worth evaluation as a surrogate endpoint in clinical trials.
MEASUREMENT OF HEALTH-RELATED QUALITY OF LIFE AND EXTRAHEPATIC OUTCOMES
NASH patients often have concomitant extrahepatic diseases, such as obesity, dyslipidemia, hypertension, T2DM, cardiovascular disease, and chronic kidney disease. In obese NASH patients, the diagnostic accuracy of noninvasive markers needs to be improved [116]. Our research group investigated the diagnostic value of metabolic disorders in NASH fibrosis [36]. Insulin resistance has been proven to play an essential role in the development of steatohepatitis and fibrosis. Although treatment may benefit comorbidities in NASH patients, there is insufficient evidence to use an improvement in metabolic comorbidities as a trial endpoint. Compared with cirrhotic patients, non-cirrhotic NASH patients are likely to have a higher incidence of cardiovascular disease [117]. In this case, metabolic-related events should be closely monitored, while longer follow-up periods are required to observe liver-related outcomes.
NAFLD not only increases the risk for development of hepatic and extrahepatic outcomes, but impairs health-related quality of life (HRQoL). In comparison with healthy controls, patients with NAFLD have decreased HRQoL scores and impaired patient-reported outcomes (PRO) that are worse than those of patients with other chronic liver diseases [118]. Changes in HRQoL and PRO scores in NAFLD are associated with hepatic disease severity and its improvement after effective treatment. The HRQoL score declines in order from NAFL to NASH, then advanced fibrosis, and cirrhosis in patients with NAFLD. Histological improvement such as reduction of steatosis degree, remission of NASH, decreased NAS, and regression of fibrosis stage after multiple new drugs trial for NASH can improve PRO and HRQoL scores. Therefore, evaluation and monitoring of HRQoL and PRO in NAFLD patients should be encouraged in routine diagnosis and treatment. PRO and HRQoL should be regarded as primary endpoints for the management of NASH and NASH-related cirrhosis.
SUMMARY
The increasing prevalence of NASH is associated with a large health economic burden globally that is characterized by excess mortality, adverse clinical outcomes, and poor patient-reported outcomes (PROs). Since there are still no effective drugs for NASH treatment, clinical trials of novel drugs have been ongoing over the past decade. NASH encompasses a heterogeneous collection of metabolic disorders and slowly progressing features of liver diseases. The challenge in monitoring NASH lies in developing techniques that allow dynamic assessment. Many noninvasive markers and algorithms to evaluate NASH severity and the efficacy of treatment have been developed. A number of serum markers, imaging modalities, and noninvasive algorithms are currently under investigation. Nevertheless, the diagnostic performance, accessibility, and cost-effectiveness of most of these modalities require improvement. Furthermore, the monitoring of NASH should also include PROs and extrahepatic diseases, especially metabolic disorders. Comprehensive but individualized surveillance should be available for each patient. We are convinced that given more efforts and cooperation among healthcare systems, researchers, pharmaceutical companies and NASH patients, advances can be made in monitoring and evaluation systems that will improve the management and prognosis of NASH patients.
Notes
Authors’ contribution
Shi YW and Fan JG contributed to the study concept and design; Shi YW and Fan JG contributed to drafting the manuscript; Fan JG contributed to critical revision of the manuscript for important intellectual content; both authors confirmed critical revision of the manuscript for important intellectual content.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
The study was funded by the National Key Research and Development Program, No. 2017YFC0908903; National Natural Science Foundation of China, No. 81900507.
Abbreviations
NASH
nonalcoholic steatohepatitis
NAFLD
nonalcoholic fatty liver disease
NAFL
nonalcoholic fatty liver
HCC
hepatocellular carcinoma
T2DM
type 2 diabetes mellitus
NFS
NAFLD fibrosis score
α-SMA
alpha-smooth muscle actin
BMI
body mass index
NAS
NAFLD activity score
ML
machine learning
AI
artificial intelligence
WSI
whole-slide images
SHG
second-harmonic generation
q-FPs
quantify fibrosis-related parameters
qFIBS
qFibrosis
FLI
fatty liver index
HSI
hepatic steatosis index
LAP
lipid accumulation product
MRS
magnetic resonance spectroscopy
DSI
Dallas Steatosis Index
FIB-4
fibrosis-4
AST
aspartate aminotransferase
APRI
AST to Platelet Ratio Index
HA
hyaluronic acid
PIIINP
amino-terminal propeptide of type III procollagen
TIMP-1
tissue inhibitor of metalloproteinase-1
NPV
negative predictive value
PPV
positive predictive value
CK18
cytokeratin 18
hs-CRP
hypersensitive C-reactive-protein
AC
attenuation coefficient
BSC
back scatter coefficient
TE
transient elastography
CAP
controlled attenuation parameter
MRI
magnetic resonance imaging
PDFF
proton density fat fraction
CT
computed tomography
LSM
liver stiffness measurement
ARFI
acoustic radiation force imaging
SSI
supersonic shear imaging
MRE
magnetic resonance elastography
MAST
MRI-aspartate aminotransferase
FAST
FibroScan-aspartate aminotransferase
HOMA
homeostasis model assessment
ADAMTSL2
A disintegrin
OCA
obeticholic acid
CPA
collagen proportionate area
HRQoL
health-related quality of life
PRO
patientreported outcomes
PROs
patient-reported outcomes