Non-invasive imaging biomarkers for liver steatosis in non-alcoholic fatty liver disease: present and future
Article information
Abstract
Non-alcoholic fatty liver disease is currently the most common chronic liver disease, affecting up to 25% of the global population. Simple fatty liver, in which fat is deposited in the liver without fibrosis, has been regarded as a benign disease in the past, but it is now known to be prognostic. In the future, more emphasis should be placed on the quantification of liver fat. Traditionally, fatty liver has been assessed by histological evaluation, which requires an invasive examination; however, technological innovations have made it possible to evaluate fatty liver by non-invasive imaging methods, such as ultrasonography, computed tomography, and magnetic resonance imaging. In addition, quantitative as well as qualitative measurements for the detection of fatty liver have become available. In this review, we summarize the currently used qualitative evaluations of fatty liver and discuss quantitative evaluations that are expected to further develop in the future.
INTRODUCTION
Metabolic syndrome has been attracting attention owing to increasing obesity, diabetes, hypertension, and lipid metabolism abnormalities resulting from the westernization of diet. The prevalence of metabolic syndrome is estimated to be 25% worldwide,¹ with similarly high and increasing rates reported from Japan² and South Korea³ in Asia. Fatty liver is known to be a frequent complication of metabolic syndrome. Fatty liver is collectively called non-alcoholic fatty liver (disease) (NAFL[D]), in which patients drink no or little alcohol (less than 30 g/day ethanol equivalent in men and less than 20 g/day in) but have a fatty liver.
The term fatty liver was first described by Thomas Addison in the 1830s in Guy’s Hospital Reports in the UK. In 1980, Ludwig proposed non-alcoholic steatohepatitis (NASH) as a condition in which a person does not drink alcohol but presents with a histology similar to an alcoholic [4]. In 1986, Schaffner first used the term NAFLD to describe the concept of fatty liver disease [5]. Subsequently, Matteoni et al. [6] published the diagnostic criteria for NASH, based on the assumption that the findings correlating with prognosis among pathological findings of NAFLD are the characteristic findings of NASH.
NAFL often has a relatively benign course, but NASH comprises a group of advanced diseases that can lead to cirrhosis and hepatocarcinoma [7]. NASH accounts for approximately 10–20% of all NAFLD cases, and is pathologically distinguished by the presence of ballooning of hepatocytes and lobular inflammation as well as fat accumulation in more than 5% of the hepatocytes [8]. Moreover, NASH and NAFL are cross connectional conditions.
Although liver biopsy is considered the gold standard for the diagnosis of fatty liver, especially in NASH, it is not practical to perform liver biopsy in all patients due to its invasiveness, potential for sampling errors, and dependency on the pathologist [9]. As Kim [10] summarized, several studies have emerged showing the use of non-invasive biomarkers to reduce the invasiveness of liver biopsy. Recently, the diagnosis of NAFLD, especially liver steatosis, has been improved by magnetic resonance imaging-proton density fat fraction (MRI-PDFF) [11] and ultrasound-controlled attenuation parameter (CAP) [12], which are increasingly recognized as possible alternatives to liver biopsy.
The recommended treatment for NAFLD is weight loss and lifestyle and exercise modifications [13]. There is still no drug that fundamentally treats NAFLD. However, there are several reports of diabetes medications being effective [14].
DEFINITION OF FATTY LIVER DISEASE AND ITS PROGNOSTIC FACTORS
Fatty liver disease is a general term for diseases that cause liver damage due to the deposition of triglycerides in hepatocytes. NAFLD is defined based on a pure ethanol equivalent intake of less than 20 g/day in women and less than 30 g/day in men. Pathologically, liver steatosis was conventionally defined as the presence of liver fat content in more than 30% of the hepatocytes; but currently, NAFLD is defined as liver fat content in more than 5% of the hepatocytes [7,15-17].
Initially, the progression from NAFL to NASH was considered a prognostic factor of NAFLD [18]. However, it has been reported that liver fibrosis is the most important prognostic factor in NAFLD, independent of the degree of liver steatosis, intralobular inflammation, and ballooning degeneration of hepatocytes, which are the findings in NASH [19-22]. It was also found that liver fibrosis progresses both in NAFL and NASH, although at different rates [23]. Therefore, the importance of assessing the degree of fibrosis, rather than diagnosing NAFL or NASH or evaluating liver steatosis, for the diagnosis of NAFLD is now recognized [23].
Since there were no comprehensive reports on the prognostic significance of NAFLD regarding the degree of liver steatosis and intralobular inflammation, simple fatty liver (NAFLD without fibrosis) development in the liver was regarded as a benign disease before 2021. Therefore, the progressive accumulation of steatosis in the liver was not recognized to have morbid implications. However, in 2021, a large Swedish cohort study showed that simple fatty liver disease, compared to the general population without fatty liver disease, was associated with a 1.9, 1.1, 7, 16.8, and 1.3 times higher risk of mortality from extrahepatic cancer, cardiovascular diseases, cirrhosis, hepatocellular carcinoma, and other causes, respectively [24], which emphasizes the importance of appropriate evaluations of liver steatosis.
Qualitative evaluations of liver steatosis have been mainly performed by abdominal sonography, computed tomography (CT) scans, and magnetic resonance imaging (MRI), but with the advent of methods such as the CAP method by FibroScan® (Echosens, Paris, France) and MRI-PDFF, it is now possible to quantify liver steatosis.
The evolution of the disease concept and evaluation methods for NAFLD/NASH are summarized in Figure 1.
ABDOMEN ULTRASONOGRAPHY (QUALITATIVE ASSESSMENT)
Abdominal ultrasonography is simple and useful for the diagnosis of fatty liver. B-mode abdominal echo findings of fatty liver include bright liver [25], hepatorenal echo contrast [26], hepatosplenic echo contrast, vascular blurring and attenuation [27], all of which are used in daily clinical practice.
B-mode findings have been reported to have good sensitivity and specificity when more than 30% of the hepatocytes have intrahepatic steatosis [28-30]. However, sensitivity and specificity are reduced when intrahepatic steatosis is less than 30% [31,32], and no studies have found that B-mode findings can diagnose less than 5% liver steatosis.
Ultrasound is a popular and useful technique for detecting fatty liver. However, ultrasonography does not provide quantitative results, and it is unsuitable for determining increases or decreases in liver steatosis and the effectiveness of treatment. In addition, it cannot detect liver steatosis under 30%, its use varies largely among surgeons; and although it is useful in diagnosing fatty liver, false-positive or -negative cases may occur [33]. At the time when abdominal ultrasound was difficult to quantify fat, a scoring system was developed to predict whether a non-drinker had NAFLD, which had a high diagnostic performance with an area under receiver operating characteristic curve (AUROC) of 0.98 based on histological evaluation [34].
ABDOMEN ULTRASONOGRAPHY (QUANTITATIVE ASSESSMENT)
The amplitude of ultrasound is attenuated exponentially as it propagates through the body. This attenuation can be broadly classified into scattering and absorption, but most of the transmitted waves on the beam are due to absorption. The attenuation constant, which represents the magnitude of attenuation, can be expressed as α=a-f n (dB/cm) as a function of frequency f in case of living tissue (the value of n is almost always 1 in soft tissue). Instead of α, attenuation can be expressed as a proportionality constant a (dB/MHz/cm). This value varies depending on the tissue and lesion type. The fact that fatty liver exhibits more attenuation than normal liver has enabled the application of quantitative ultrasonography for liver steatosis.
CONTROLLED ATTENUATION PARAMETER (CAP)
FibroScan® (EchoSens, Paris, France), the pioneering instrument in vibration controlled transient elastography, was introduced in 2003 [35]. Initially, it could only measure liver stiffness, but in 2010, CAP was introduced to measure the degree of fat attenuation [36]. This was the first time that a device was able to quantify liver steatosis. Although the CAP method was considered non-invasive, rapid, inexpensive, and reproducible, it was less suitable for obese patients, in whom acquiring ultrasound signals was difficult with the available M probe. However, with the introduction of the XL probe for obese patients [37], shear waves are now able to penetrate deeper and generate signals in obese patients as well. The XL probe was also equipped with CAP, making it more useful for measurements in obese patients [38].
In 2021, EchoSens launched the new computation method SmartExam allowing for deeper measurements and an increased number of CAP measurements, which is expected to further improve the accuracy of CAP measurements in obese patients [39]. Owing to its recency, there are few reports on this method, but further studies are in progress. Recently, we presented the first clinical report on the SmartExame-quipped FibroScan [40]. In our study, we compared the SmartExam-equipped FibroScan and the conventional FibroScan with the results obtained with magnetic resonance imaging (MRE)/MRI-PDFF, and reported that both are capable of comparable evaluation. We also concluded that the SmartExame-quipped FibroScan significantly reduced CAP variability, but tended to take slightly longer to obtain measurements compared to the conventional FibroScan. One limitation of this paper was the small number of obese patients, and further studies in a population with a large number of obese patients was recommended.
A meta-analysis of the diagnostic performance of CAP based on histological evaluation by liver biopsy in NAFLD showed high AUROCs of 0.924, 0,784, and 0.778 for S ≥1, 2, and 3, respectively [41]. The usefulness of CAP is emphasized in various NAFLD guidelines, including the American Association for the study of Liver (AASLD) [8], European Association for the study of the liver (EASL) [42], the Korean Association for the Study of the liver (KASL) [43], and Japanese guidelines [16,17].
The advantage of CAP is that fatty liver quantification can be performed easily, quickly, and inexpensively with high diagnostic performance. However, the disadvantage is that the measurement results are affected by the distance to the liver surface making it necessary to change the probe to M or XL depending on advanced obesity and body size [38]. Different probes have different transmission frequencies; thus, resulting values cannot be simply compared. In addition, CAP measurements cannot be performed in cases of ascites or effusion, but some newer techniques have overcome such drawbacks.
Furthermore, it has been reported that liver stiffness measurements using FibroScan® are useful in assessing liver fibrosis in long-term follow-up [44,45]. However, it has not been reported whether the measurement of liver steatosis is also useful in long-term follow-up, and we hope that such studies are conducted in the future.
OTHER UPCOMING ULTRASOUND-BASED QUANTITATIVE EVALUATION METHODS
Since the advent of CAP, devices that measure attenuation coefficients simultaneously with B-mode images on conventional abdominal ultrasound systems have been developed and put into practical use, including UGAP (GE Healthcare, Wauwatosa, WI, USA), ATI (Canon Medical Systems, Tochigi, Japan), Attenuation Imaging (Fujifilm Healthcare, Tokyo, Japan) [46], ultrasound-derived fat fraction (UDFF) (Siemens Healthineers, Erlangen, Germany) [47-49], attenuation estimation algorithm (Hologic, Bedford, MA, USA), tissue-attenuation imaging (Samsung Medison, Seoul, Korea), and Philips attenuation (Philips Medical Systems, Amsterdam, The Netherlands).
ATTENUATION IMAGING (ATI)
ATI can also measure liver fat content without changing the probe. The principle of ATI is that it can avoid multiple reflections from a close range, which has been a disadvantage in diagnosis. It also eliminates the focal point dependence of the transmitted sound field characteristics, deep attenuation, and large vessels, which are dependent on the probe and affect the measured value, and it can automatically calculate and quantitatively evaluate the attenuation due to the properties of biological tissue in any part of the body. In addition, it is possible to automatically calculate and quantitatively evaluate the attenuation rate caused by the characteristics of the biological tissue in any part of the body. ATI has been reported to have as high diagnostic performance as MRI-PDFF in terms of liver fat quantification compared to MRI-PDFF [50-61]. It is reported that ATI has good correlation with CAP (r=0.65, P<0.0001) and the AUROC for detecting S >0 steatosis and S >1 steatosis was 0.91 and 0.88, respectively [52]. Tada et al. [50] also reported that ATI-induced attenuation coefficient values are not affected by liver stiffness.
As for ATI, it has only been studied on a small scale and is expected to be studied on a larger scale in the future. The advantage of ATI is that it has a high diagnostic performance and, unlike CAP, can be measured in the presence of ascites. It is also advantageous that the same machine can perform measurements while observing in B-mode. On the other hand, ATI is less commonly reported and less widely used than CAP.
ULTRASOUND-GUIDED ATTENUATION PARAMETER (UGAP)
UGAP is a fat quantification method based on measuring the attenuation coefficient (dB/cm/MHz) of the ultrasound signal in the common B mode. It was first reported in 2018 by Fujiwara et al. [62], and was shown to be comparable in terms of AUROC to CAP and MRI-PDFF, the latter being considered an alternative to liver biopsy for the evaluation of liver steatosis with comparable diagnostic performance, as shown in a multicenter study [63]. In this study, the AUROCs of UGAP for distinguishing steatosis grade ≥1 (MRI-PDFF ≥5.2%), ≥2 (MRI-PDFF ≥11.3%), and 3 (MRI-PDFF ≥17.1%) were 0.910 (95% confidence interval [95% CI], 0.891–0.928), 0.912 (95% CI, 0.894–0.929), and 0.894 (95% CI, 0.873–0.916), respectively, showing an excellent diagnostic accuracy for grading steatosis with reference to MRI-PDFF. The advantages and disadvantages of UGAP are similar to those of ATI. There have been a few reports, but further evaluations are expected.
Several new ultrasound techniques for measuring liver steatosis from various companies, including improved version of the attenuation coefficient (iATT) and UDFF, have been introduced, but they are still lacking evidence.
Table 1 summarizes the modalities and standard references for liver steatosis reported to date, and Table 2 summarizes the AUROCs of non-invasive imaging modalities.
STEATOSIS QUANTIFICATION AND QUALIFICATION USING CT
A comparison of CT values of the liver and spleen (liver/spleen ratio: L/S ratio) [64,65] is useful for the early detection of fatty liver. When the CT values of the liver are lower than those of the spleen due to increased fat accumulation in the liver, a fatty liver can be diagnosed. However, CT scans are costly and time-consuming; thus, a rapid and more readily available means of assessing NAFLD in routine clinical care is needed [66]. Unlike ultrasound and MRI, CT is now used less frequently due to exposure issues, its low quantitative nature, and its relatively poor performance in detecting mild steatosis and quantifying steatosis [67-69].
Dual energy CT is a quantitative imaging method that uses two different X-ray tube voltages to estimate the composition of an imaging target using a material decomposition method that utilizes material-specific X-ray absorption characteristics.
Since the 1990s, reports on liver fat evaluation using dual energy CT have been published [70,71]. Using MRI-PDFF >6% as a reference diagnosis of fatty obesity, the diagnostic performance of fatty liver using dual energy CT was reported with an AUROC of 0.834. Optimal thresholds were 54.8 hounsfield unit (HU) (right) and 52.5 HU (left), with sensitivities/specificities of 57%/93.9% (right) and 67.9%/90% (left). For the hepatosplenic weight loss difference, the AUROCs were 0.808 (right) and 0.767 (left), with optimal sensitivities/specificities of 93.3%/57.1% (right) and 78.6%/68% (left) [72].
It has been suggested that positron emission tomographycomputed tomography may be used in the future. Liver steatosis in NAFLD patients is independently associated with elevated liver enzymes, increased visceral adipose tissue volume, and decreased myocardial fluorodeoxyglucose-positron emission (FDG) uptake, but not with hepatic FDG uptake [73]. These properties could allow the clinical use of positron emission tomography—computed tomography for liver fat mass quantification in the future.
STEATOSIS QUANTIFICATION USING MRI
MRI signals are obtained from protons belonging to water and fat molecules, making it a good method for quantifying fat in the liver.
Proton magnetic resonance spectroscopy has been shown to be a safe and non-invasive method of quantifying liver fat content that correlates well with liver biopsy [74-78], and can detect fat depositions as little as 2% [79]. However, it has not been widely adopted in general clinical practice, partly, due to specific software requirements [80].
Subsequently, MRI-PDFF was introduced, which is a technique that allows the assessment of the amount of fat in the entire liver or in arbitrary regions of interest, even in small amounts [81,82]. Recently, studies have used MRI-PDFF instead of liver biopsy as a reference standard [50-60,63,78-83]. It has been reported that MRI-PDFF measurements correlate strongly with histological liver fattening [84,85]. In a comparison of pathological findings, the AUROC had an extremely high diagnostic accuracy of 0.99 for predicting hepatic steatosis by MRI-PDFF, which was much higher than that of CAP (AUROC 0.85) [86].
The AASLD [8], KASL [43], and Japanese guidelines [16,17] also emphasize the usefulness of MRI-PDFF. In addition to quantifying liver steatosis in clinical practice, recent clinical trials on NAFLD have examined histological evaluation, MRI-PDFF, and CAP reduction rates to investigate whether liver steatosis improves before and after investigational drug treatment [87]. According to a recent review on quantitative liver steatosis assessment, MRI-PDFF should be used as a non-invasive reference standard in diagnostic studies [46].
APPLICATION OF ARTIFICIAL INTELLIGENCE IN THE MEASUREMENT OF LIVER STEATOSIS
In recent years, artificial intelligence (AI) has been utilized in many fields. AI software tries to reproduce human logical thinking on a computer. With the development of deep learning technology, AI can autonomously learn and construct decision criteria from given data. The fields of pathology and imaging evaluation have a high affinity to AI which has enabled remarkable technological developments for clinical applications.
The advantages of AI are that it continuously provides stable results as it does not suffer from the exhaustion that occurs in humans, and that it prevents inter- and intra-observer variability. It has been reported that AI technology minimizes inter-observer variability in histological assessments [88,89]. Among other things, AI technology has the potential for the objective assessment of ballooning, which is a hallmark in the evaluation of NAFLD steatosis [90].
Reports have also been published on AI-assisted ultrasound and MRI, which are expected to be useful in clinical practice. A meta-analysis on liver steatosis using AI technology was published by Decharatanachart et al. [91]. They summarized 19 previous studies that assessed fibrosis and steatosis of the liver using AI-based ultrasound, elastography, CT, MRI, and clinical parameters. According to the pooled data, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic odds ratio (DOR) for the diagnosis of liver steatosis were 0.97 (0.76–1.00), 0.91 (0.78–0.97), 0.95 (0.87–0.98), 0.93 (0.80–0.98), and 191.52 (38.82–944.81), respectively. AI technology is expected to be used in clinical practice in the future.
New concept, metabolic associated fatty liver disease (MAFLD)
It is known that fatty liver can occur whether one drinks alcohol or not; and since it is often complicated by lifestyle-related diseases, it has been proposed that fatty liver should be considered a MAFLD going forward, and not NAFLD [92,93].
CONCLUSIONS
Fat content in NAFLD is nowadays evaluated quantitatively as well as qualitatively. Although histological evaluation remains the gold standard for liver steatosis measurement, it is likely to be replaced by MRI-PDFF in the future. Once additional evidence on the usefulness of fat determination by ultrasound using novel technology becomes available, liver fat content could potentially be measured easier than ever before in general clinical practice. Several methods have emerged to quantify liver steatosis, but each test has its own advantages and disadvantages in terms of diagnostic performance, cost, and invasiveness (Fig. 2).
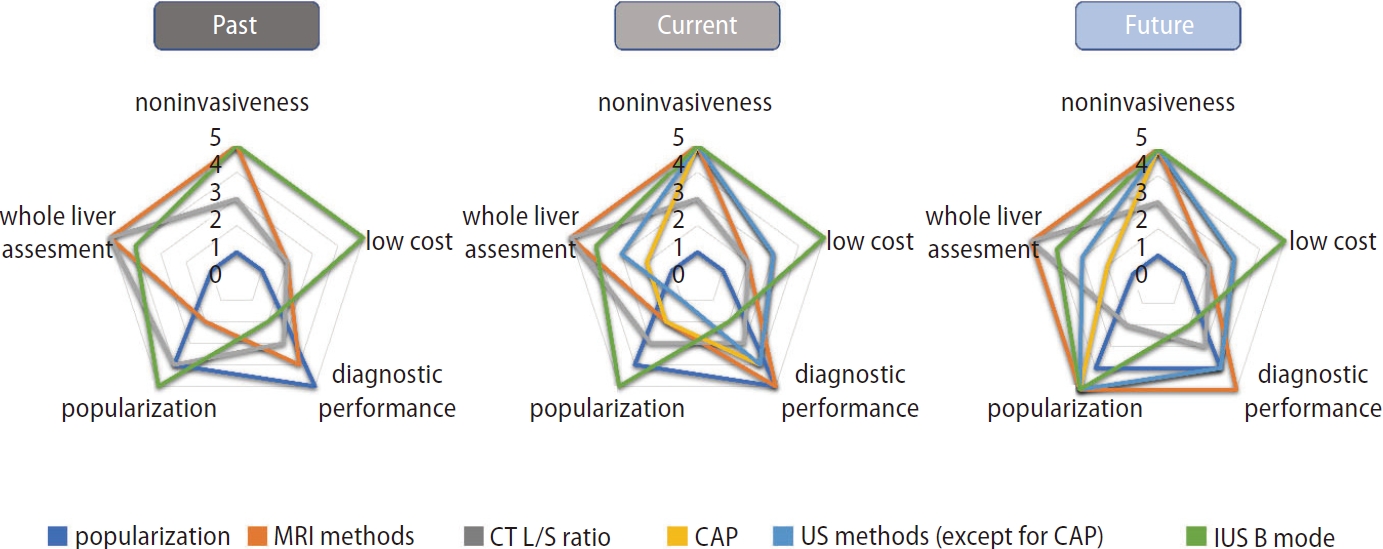
Characteristics of examinations to evaluate liver steatosis in the past, present, and future. MRI, magnetic resonance imaging; CT, computed tomography; CAP, controlled attenuation parameter; US, ultrasound; L/S ratio, liver-to-spleen ratio.
Various liver steatosis measurement techniques are now available. However, the coherence between these techniques remains unclear. Further evidence and additional clinical studies are required.
Notes
Authors’ contribution
AN wrote the manuscript and prepared the figures and tables. MY and AN revised the manuscript. All the authors read and approved the final version.
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
AASLD
American Association for the study of Liver
AI
artificial intelligence
AUROC
area under receiver operating characteristic curve
ATI
attenuation imaging
CAP
controlled attenuation parameter
CLD
chronic liver disease
CT
computed tomography
EASL
European Association for the study of the liver
KASL
Korean Associatoin for the Study of the liver
MRI
magnetic resonance imaging
MRI-PDFF
magnetic resonance imaging-proton density fat fraction
NAFL
non-alcoholic fatty liver
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
UGAP
ultrasound-guided attenuation parameter



