Non-invasive tests-based risk stratification: Baveno VII and beyond
Article information
Portal hypertension is the main driver of complications in compensated advanced chronic liver disease (cACLD). Particularly, the presence of clinically significant portal hypertension (CSPH) identifies those at risk for hepatic decompensation [1].
The recommendation of the recent Baveno VII consensus to treat CSPH upon diagnosis (i.e., prevention of hepatic decompensation), instead of waiting for (high-risk) varices to develop to initiate primary bleeding prophylaxis, marked a paradigm shift in clinical hepatology [2]. Specifically, endoscopies to screen for high-risk varices or performance of endoscopic band ligation have been a cornerstone in the management of cACLD patients (i.e., primary prophylaxis of variceal bleeding) until recently [3,4]. Following Baveno VII [2], the presence of CSPH should be investigated by non-invasive tests (NIT) [5] or, where available, hepatic venous pressure gradient-measurement [6] upon the diagnosis of cACLD, to facilitate timely treatment initiation with non-selective betablockers (NSBB; preferably carvedilol) for preventing first hepatic decompensation [1,7,8] - most commonly, the occurrence of ascites [2].
In a recent issue of Clinical and Molecular Hepatology, Wong and colleagues [9] set out to validate the performance of NIT to exclude CSPH (liver stiffness measurement [LSM] <15 kPa and platelet count [PLT] ≥150×109/L), rule-in CSPH (LSM ≥25 kPa), and to identify those at high probability for CSPH (LSM 20–25 kPa and PLT <150×109/L, or LSM 15–20 kPa and PLT <110×109/L) for the prediction of first hepatic decompensation in a multicentre cohort, including 1,159 ‘cACLD’ patients from Italy, India, China, and Singapore. Notably, this multi-ethnic cohort included predominantly cured hepatitis C virus (HCV) (56%) or suppressed hepatitis B virus (HBV) (21%) patients; the median LSM was approximately 24 kPa at study inclusion, and the patients were followed for 40 months.
The authors report on several important aspects: patients in whom CSPH could be ruled-in (LSM ≥25 kPa; 37% of the study population) had a substantially increased risk of first hepatic decompensation within the follow-up period across all aetiologies (14% at 3 years). At the same time, patients in whom CSPH could be excluded (LSM <15 kPa and PLT ≥150×109/L) did not develop any hepatic decompensation during follow-up, yet a considerable proportion developed hepatocellular carcinoma. However, patients within the diagnostic/prognostic ‘grey-zone’ (i.e., not falling into one of these categories; 51% in this study) still had a relevant risk to develop first hepatic decompensation, especially in non-viral aetiologies. Also, using the selected criteria for a ‘high probability of CSPH’ within this grey-zone did not introduce any granularity, and was, therefore, insufficient for prognostication.
These data clearly support the utility of NIT-based criteria to rule-in or exclude CSPH and stratify the risk of its clinical sequalae in everyday practice, as they identify subsets of patients with profoundly different risks of first hepatic decompensation. However, a large proportion of patients (>50%) fall into neither category, leaving them unclassified. Here, recent approaches have introduced spleen stiffness measurement (SSM) [10] or the ratio of von Willebrand factor (VWF) and PLT (VITRO score) [11] to close this diagnostic gap (Fig. 1; 1st scenario) [12,13]. Specifically, combining Baveno VII criteria (as outlined above) with SSM ≤40 kPa/>40 kPa [12], or sequentially applying Baveno VII criteria and a VITRO-score ≤1.5 or ≥2.5 reallocated up to 75% of previously unclassified patients into the ruled-out/in category while maintaining a high diagnostic accuracy, thereby reducing the grey-zone for CSPH to only 10–15% of all cACLD patients [12,13]. Most importantly, both SSM- and VITRO-based approached were also able to discriminate between patients at risk vs. those not at risk for first hepatic decompensation [12,13]. The sequential application might especially be important to identify ‘at-risk’ patients with CSPH who are otherwise missed by LSM alone. While the ≥25 kPa cut-off is generally endorsed across all etiologies, the optimal cut-off for CSPH might vary across etiologies [14-16], prompting other NIT, such as SSM/VWF/VITRO, that better reflect the dynamic component of portal hypertension [17].
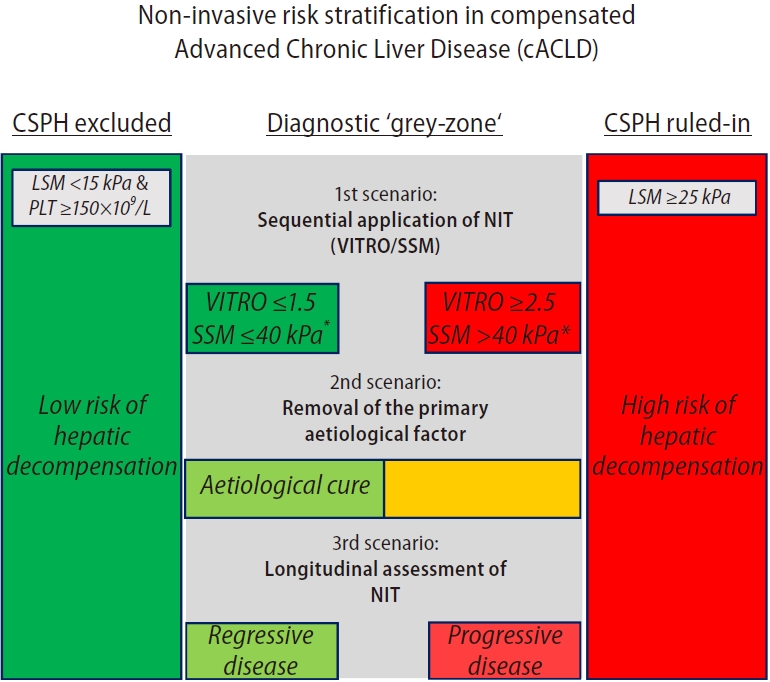
Three different approaches (i.e., scenarios) to improve risk stratification in the diagnostic grey-zone of clinically significant portal hypertension (CSPH). LSM, liver stiffness measurement; PLT, platelet count; NIT, non-invasive tests; VITRO, ratio of von Willebrand factor and platelet count; SSM, spleen stiffness measurement. *Modified from Dajti et al. (2022) requesting 2 of 3 criteria to exclude CSPH (LSM <15 kPa and PLT ≥150×109/L or SSM ≤40 kPa) and 2 of 3 to rule-in CSPH (LSM ≥25 kPa and PLT <150×109/L or SSM >40 kPa).
In general, the inclusion of ‘cACLD’ patients in whom the primary aetiological factor has been removed (i.e., HCV, 56%; alcoholic liver disease [ALD], 9%, patients with reported ongoing abuse were excluded) or suppressed (HBV, 21%) as done by Wong and colleagues [9] might indeed be reflective of todays ‘real-world’ practice, yet it introduces heterogeneity in the underlying risk for first hepatic decompensation. Here, numerous studies have shown that the risk of first hepatic decompensation is considerably lower after HCV-cure [18], and that specific risk stratification algorithms for CSPH [19] but also first hepatic decompensation (e.g., by combining LSM and VITRO) are required [20,21] while the overall accuracy of NIT for CSPH is generally comparable [22]. To account for these peculiarities, the term ‘cACLD’ as currently defined by Baveno VII even explicitly excludes patients after removal of the primary aetiological factor (‘the term cACLD had been proposed to reflect the continuum of severe fibrosis and cirrhosis in patients with ongoing chronic liver disease’ [2])–which was not accounted for in this study.
Most interestingly, non-viral aetiology (which basically reflects the absence of ‘removal of the primary aetiological factor’) had a stronger impact on first hepatic decompensation risk as compared to having a high probability of CSPH (i.e., the disease stage) at baseline (subdistribution hazard ratio, 3.25 vs. 2.48). This underscores the profound change in underlying risk achieved by aetiological cure, as it already provides a glimpse into the likely future of the patient (i.e., progressive vs. regressive disease). At the same time, this strongly argues for the incorporation of the concept of ‘aetiology’/‘removal of the primary aetiological factor’ into risk stratification models (Fig. 1; 2nd scenario). Alternatively, NITs offer the unique opportunity to longitudinally monitor disease dynamics (progression vs. regression), and therefore, repeated re-staging (Fig. 1; 3rd scenario). Here, it remains to be shown whether the underlying risk may be even better captured by repeating NITs (i.e., NIT trajectories) and whether the consideration of these trajectories outperforms concepts of ‘aetiology’/‘removal of the primary aetiological factor’ alone.
Importantly, patients with NSBB treatment at baseline were excluded from this study. However, this may underestimate the risk of first hepatic decompensation among CSPH ruledin patients encountered (but not scoped) in recent clinical practice, as patients with high-risk varices (and thus, most severe portal hypertension/highest decompensation risk) were underrepresented by design. Also, patients were not censored at the time of the initiation of prophylactic treatment (in particular, NSBB therapy), which may also have decreased the first hepatic decompensation risk.
Compared to the “PREDESCI” study, and as discussed by the authors, the risk of first hepatic decompensation was considerably lower in the study by Wong and colleagues [9] (24% in the placebo group of the “PREDESCI” study vs. 13.3% of CSPH ruled-in patients from this study) [23]. While this can be explained by differences in the underlying patient population (patients with CSPH vs. CSPH ruled-in by NIT; only active HCV infection in the PREDESCI trial vs. only cured HCV patients in the present study), it also influences the number-needed-to-treat (NNT) for NSBB, which might be even lower than in the study by Wong and colleagues [9] (proposed NNT of 27–50). This calls for a ‘non-invasive’ PREDESCI trial to re-ensure our current clinical practice using contemporary patients.
Finally, it remains unclear how regional differences in healthcare might have confounded the results of our study, as aetiologies showed profound geographical clustering: 59%/27% of all HCV patients were treated in Italy/Singapore; 57% of all HBV patients were treated in China, 86% of all ALD patients were treated in India; and 68% of all NASH-patients were treated in India. Since including patients from around the globe does not guarantee the generalizability of the findings to a specific region, evaluating geographical regions/aetiologies independently might be another important task for future studies.
All things considered, the study by Wong et al. [9] is an important proof-of-concept, indicating that non-invasive risk stratification for CSPH is valid across different aetiologies, ethnicities, and countries, as it identifies patients at risk for first hepatic decompensation who may benefit from NSBB treatment (CSPH ruled-in), and those at negligible risk of hepatic decompensation (CSPH excluded). More granular information is required to optimize risk stratification/treatment allocation in the broad diagnostic grey-zone of the Baveno VII recommendations; however, specifically designed NIT-based approaches (SSM [12] and VITRO [13]) have already been added to our armamentarium. Finally, a randomized controlled trial would be desirable to provide a definite proof for the Baveno VII approach to use NSBB treatment to prevent first hepatic decompensation in patients in whom CSPH has been ruledin non-invasively.
Notes
Authors’ contribution
Drafting of the manuscript (G.S., M.J., M.M.), critical revision of the manuscript for important intellectual content (G.S., M.J., M.M.).
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
ALD
alcoholic liver disease
cACLD
compensated advanced chronic liver disease
CSPH
clinically significant portal hypertension
HBV
hepatitis B virus
HCV
hepatitis C virus
LSM
liver stiffness measurement
NIT
non-invasive tests
NNT
number-needed-to-treat
NSBB
non-selective beta blocker
PLT
platelet count
SSM
spleen stiffness measurement
VITRO
ratio of von Willebrand factor and platelet count
VWF
von Willebrand factor
