Old and new classes of glucose-lowering agents as treatments for non-alcoholic fatty liver disease: A narrative review
Article information
Abstract
Non-alcoholic fatty liver disease (NAFLD) has become the most common chronic liver disease with a global prevalence of about 55% in people with type 2 diabetes mellitus (T2DM). T2DM, obesity and NAFLD are three closely inter-related pathological conditions. In addition, T2DM is one of the strongest clinical risk factors for the faster progression of NAFLD to non-alcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma. Increasing evidence suggests that newer classes of glucose-lowering drugs, such as peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase-4 inhibitors or sodium-glucose cotransporter-2 inhibitors, could reduce the rates of NAFLD progression. This narrative review aims to briefly summarize the recent results from randomized controlled trials testing the efficacy and safety of old and new glucose-lowering drugs for the treatment of NAFLD or NASH in adults both with and without coexisting T2DM.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD), defined as fat accumulation in the hepatocytes in individuals without excessive alcohol consumption, has become a potentially serious global chronic liver disease, affecting up to nearly 30% of the adult population worldwide [1-3]. NAFLD is a histological spectrum of progressive liver conditions ranging from NAFL to non-alcoholic steatohepatitis (NASH), advanced fibrosis and cirrhosis [4-8]. To date, there is no approved pharmacotherapy for NAFLD or NASH. Thus, there is a critical need to identify effective pharmacological treatments to prevent and treat this common and burdensome liver disease.
NAFLD occurs with metabolic dysfunction that is closely associated with overweight/obesity, insulin resistance, and type 2 diabetes mellitus (T2DM) [9,10]. More than 55% of patients with T2DM have NAFLD [10,11], and patients with T2DM are also more likely to develop the more advanced forms of NAFLD (e.g., NASH, cirrhosis or hepatocellular carcinoma) [12-15]. T2DM and NAFLD are two pathological conditions that act synergistically to increase the risk of adverse clinical outcomes through complicated pathophysiological mechanisms, such as insulin resistance, chronic hyperglycemia, lipotoxicity, low-grade inflammation, and increased oxidative stress [10,14,16]. As early as 2016, the European Association for the Study of the Liver, the European Association for the Study of Diabetes and the European Association for the Study of Obesity societies strongly recommended screening for NAFLD in patients with established T2DM and screening for T2DM in patients with NAFLD [17]. Furthermore, an international panel of experts recently proposed a re-definition and re-classification of NAFLD, as metabolic dysfunction-associated fatty liver disease (MAFLD) [18-21]. It has been proposed that the MAFLD definition may help facilitate a better understanding of metabolic factors involved in the development of NAFLD and T2DM, which are two closely inter-related pathological conditions [18-21]. The current definition of NAFLD requires the exclusion of significant alcohol consumption and other secondary causes of hepatic steatosis. In contrast, the newly proposed definition of MAFLD is not an exclusionary diagnosis, and is based on the evidence of hepatic steatosis (as assessed by liver biopsy or imaging techniques) and the coexistence of at least one of the following three metabolic risk factors: 1) overweight or obesity; 2) established T2DM; or 3) metabolic dysregulation [22]. MAFLD may therefore be a more suitable terminology to describe this common and burdensome liver disease that is closely related to underlying metabolic dysfunction. MAFLD may also be a more accurate definition of ‘NAFLD’ in patients where fatty liver disease coexists with T2DM, and where patients are at increased risk of developing extra-hepatic complications, such as cardiovascular disease (i.e., the leading cause of death in people with NAFLD), certain types of extra-hepatic cancers, chronic pulmonary and renal diseases [23-28].
Despite intensive research, there is still no drug to date that has been approved for the treatment of NAFLD or NASH. Lifestyle modifications, which include hypocaloric diet and physical activity to achieve weight loss, are the cornerstone of treatment for NAFLD and NASH [6,17,29]. Although lifestyle modifications are effective for nonalcoholic simple steatosis and early NASH, they have limited efficacy in reversing liver fibrosis, particularly in patients with NASH and T2DM [10,30]. In contrast to the smaller body weight reductions obtainable by traditional lifestyle change approaches, a recent study showed the possibility of obtaining regression of liver fibrosis in severely obese patients with NASH after gastric bypass surgery [31]. Therefore, these findings highlight the need for drugs that may prevent or reverse NAFLD or NASH to solve this global health problem. Nevertheless, some newer glucose-lowering drugs that are widely used for the treatment of T2DM, such as peroxisome proliferator-activated receptor (PPAR) agonists, including thiazolidinediones (TZDs), glucagon-like peptide-1 receptor agonists (GLP-1RAs), dipeptidyl peptidase-4 (DPP-4) inhibitors or sodium-glucose cotransporter-2 (SGLT-2) inhibitors, have shown promising results for the treatment of NAFLD and NASH [32-37].
We therefore carried out an updated narrative review to briefly summarize the efficacy and safety of the aforementioned newer glucose-lowering drugs in adults with NAFLD or NASH. The results of principal randomized clinical trials examining the efficacy of these drugs for specifically treating adults with biopsy-proven NASH, regardless of the presence or absence of T2DM, are summarized in Table 1. The main putative mechanisms for diabetes-induced NAFLD are schematically illustrated in Figure 1, whereas the putative underlying mechanisms by which these glucose-lowering drugs may exert their possible hepato-protective effects are shown in Figures 2, 3.
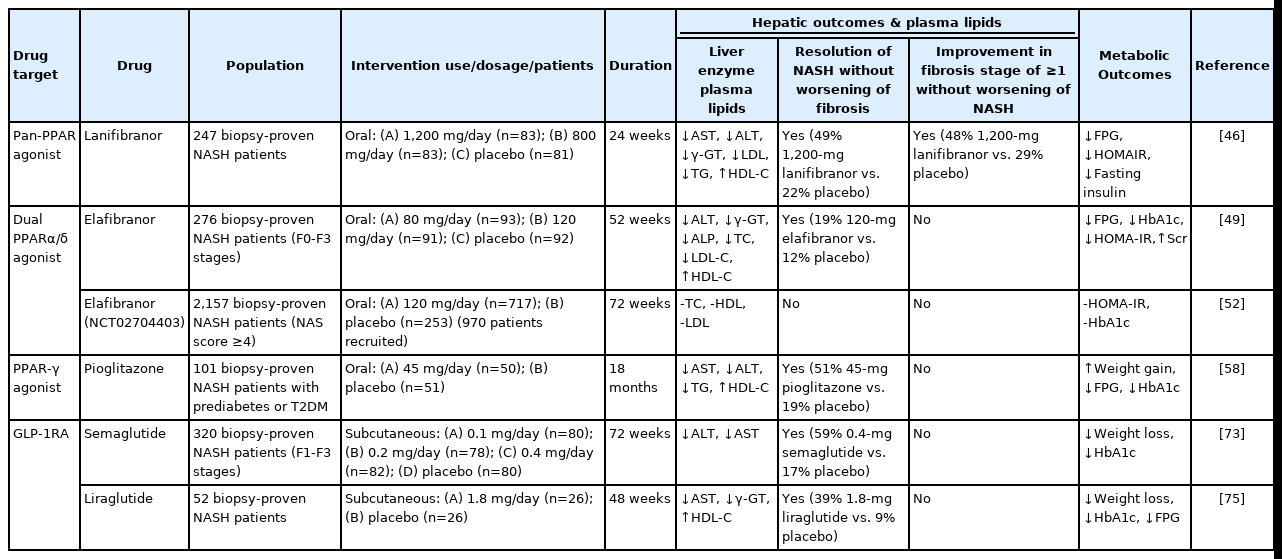
Old and new glucose-lowering agents as potential treatments for adult patients with biopsy-proven NASH
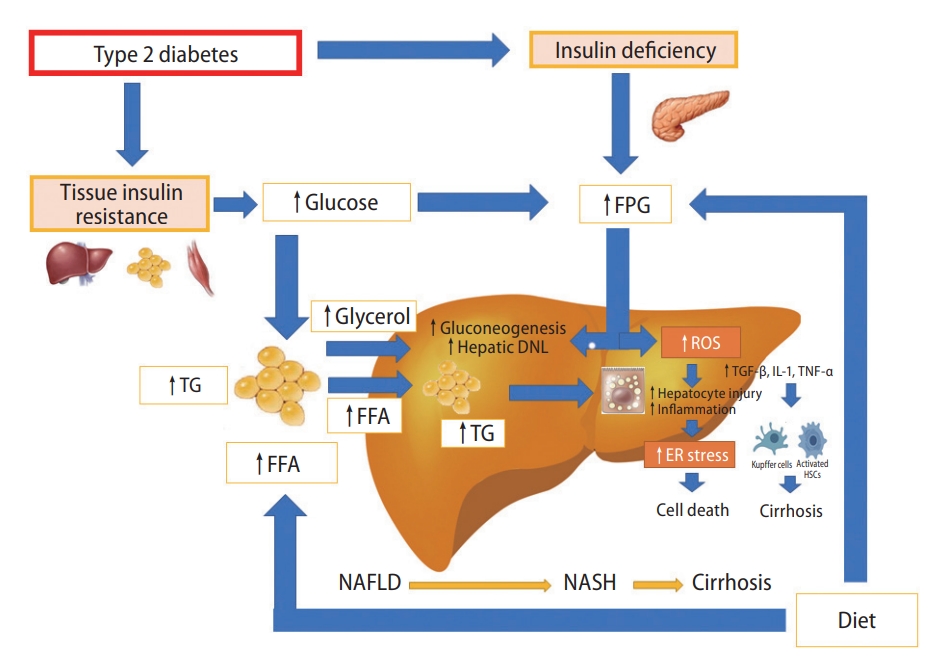
This schematic diagram illustrates the main mechanisms of diabetes-induced NAFLD. With type 2 diabetes there is usually insulin resistance, reduced pancreatic beta-cell insulin secretion and chronic hyperglycaemia. Adipose tissue lipolysis provides a source of FFA and saturated and monounsaturated fatty acids that are a powerful substrate and stimulus for hepatic DNL. Release of glycerol from lipolysis also provides a substrate for hepatic gluconeogenesis. With hepatic insulin resistance and high levels of glucagon, there is a further increase in gluconeogenesis and a relative decrease in insulin-mediated suppression of hepatic glucose production that further promote fatty liver. In this context, the progression of NAFLD to NASH and cirrhosis is mainly due to increased production of ROS, which leads to ER stress, release of proinflammatory cytokines, cell death and increased fibrogenesis by hepatic stellate cells. FPG, fasting plasma glucose; TG, triglycerides; FFA, free fatty acids; DNL, de novo lipogenesis; ROS, reactive oxygen species; TGF, transforming growth factor; IL, interleukin; TNF, tumor necrosis factor; ER, endoplasmic reticulum; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis.

This schematic diagram illustrates the key cellular targets of different PPARs for the treatment of NASH and fibrosis whose modulation is intended mainly to reduce hepatic fat content, improve insulin resistance and glucose homeostasis, reduce low-grade inflammation, as well as improve mitochondrial function of hepatocytes and reduce fibrogenesis by hepatic stellate cells. FFA, free fatty acids; PPAR, peroxisome proliferator-activated receptor; FAO, fatty acid oxidation; FPG, fasting plasma glucose; ROS, reactive oxygen species; DNL, de novo lipogenesis; HSCs, hepatic stellate cells; ER, endoplasmic reticulum; NASH, non-alcoholic steatohepatitis.

his schematic diagram illustrates the targets of GLP-1RAs, DPP-4 inhibitors and SGLT-2 inhibitors for the treatment of NASH whose modulation is intended mainly to reduce hepatic fat content, improve insulin resistance and glucose homeostasis. GLP, glucagon-like peptide; GIP, glucose-dependent insulinotropic polypeptid; GLP-1RA, glucagon-like peptide-1 receptor agonist; DPP-4, dipeptidyl peptidase-4; FFA, free fatty acids; FPG, fasting plasma glucose; SGLT-2, sodium-glucose cotransporter-2; HSCs, hepatic stellate cells; ROS, reactive oxygen species; DNL, de novo lipogenesis; FAO, fatty acid oxidation; ER, endoplasmic reticulum; NASH, non-alcoholic steatohepatitis.
PROMISING GLUCOSE-LOWERING DRUGS FOR NAFLD AND NASH
PPAR agonists
PPAR is a nuclear receptor activated by different ligands that plays a key role not only in fatty acid and lipid metabolism, but also in glucose homeostasis, low-grade inflammation and fibrogenesis. These effects make PPARs an attractive therapeutic target for the treatment of NAFLD and NASH [38,39]. Recently, many studies reported significant improvements of the individual histological components of NASH, resolution of NASH or regression of fibrosis with the use of the PPAR-agonist pioglitazone [34,38].
There are several PPARs: PPAR-α, PPAR-β/δ and PPAR-γ. PPAR-α is a key regulator of fatty acid oxidation which occurs in the liver, skeletal muscle and adipose tissue. PPAR-α suppresses inflammation mainly through the reduction of reactive oxygen species production, improves plasma lipid profile and participates in the regulation of energy homeostasis [40]. PPAR-β/δ activates the pathways of hepatic glucose utilization and de novo lipogenesis, promotes hepatic fat oxidation, regulates innate immunity and reduces inflammation [41]. PPAR-γ, which is activated by TZDs, is highly expressed in adipose tissue as the PPAR-γ2 isoform, and plays a role in the regulation of adipocyte differentiation, insulin resistance, adipogenesis, and lipid metabolism [42,43].
Lanifibranor
Lanifibranor (IVA337) is a first-in-class pan-PPAR agonist with the ability to activate three PPAR isotypes (α, γ, δ) [44,45]. Recently, in a phase 2b placebo-controlled randomized clinical trial testing the efficacy of lanifibranor in NASH (NCT01694849, the NATIVE trial), 247 obese patients with biopsy-proven NASH were randomly assigned to three treatment arms: 83 patients received 1,200-mg lanifibranor daily, 83 of patients received 800-mg lanifibranor daily, and 81 patients received placebo for 24 weeks. The primary endpoint was the improvement of at least two points in the histologic Steatosis, Activity and Fibrosis (SAF)-score without worsening of fibrosis, whereas the secondary endpoints were resolution of NASH and regression of liver fibrosis. The results of this trial showed that the 1,200-mg dose of lanifibranor significantly decreased SAF-score by at least 2 points without worsening of fibrosis in 55% patients vs. 33% in placebo. Treatment with lanifibranor also resulted in significant reductions of serum liver enzymes, plasma lipids, proinflammatory biomarkers and fibrosis test scores [46]. Side effects of 24-week treatment with lanifibranor included diarrhea, nausea, peripheral edema, anemia and weight gain, a part of which were very similar to those observed with pioglitazone use. Thus, it remains debatable whether the benefits of lanifibranor on NASH histology are mainly related to its PPAR-γ effects, and more research is needed to clarify this issue [45-48]. Although there were no life threatening side effects observed in the lanifibranor group; nausea (~8%), diarrhea (12%), fatigue (13%), peripheral edema (2%), anemia (7%), and weight gain (3%) occurred more frequently in the 1,200-mg dose lanifibranor group than in the placebo group. That said, if the results of the NATIVE trial are confirmed in larger phase 3 randomized clinical trials, it is reasonable to assume that lanifibranor will become one of most promising treatment options for NASH.
Elafibranor
Elafibranor (GFT505) is a dual PPAR-α/δ agonist sharing structural similarities to other well-known PPAR-γ agonists, and elafibranor effects the regulation of many metabolic processes, including aiding the decrease of inflammatory properties and dyslipidaemia, as well as providing a protective effect on the risk of major cardiovascular events [49-51]. In a phase 2b randomized placebo-controlled trial (NCT01694849), 276 overweight or obese patients with biopsy-proven NASH were randomly assigned to three treatment arms: 93 patients received 80-mg elafibranor daily, 91 of patients received 120-mg elafibranor daily, and 92 patients received placebo for 52 weeks. In the intention-to-treat analysis, there was no significant difference between the elafibranor and placebo groups in the protocol-defined primary outcome of NASH resolution without worsening of fibrosis. However, based on a post-hoc analysis, the authors found the 120-mg elafibranor dose was associated with an improvement in 2 points in NAFLD activity score (48% elafibranor vs. 21% placebo; P=0.013) and without worsening of fibrosis (20% elafibranor vs. 11% placebo; P=0.018). Furthermore, serum liver enzymes, lipids, glycemic control, and proinflammatory markers were also improved in the 120-mg elafibranor group [49]. Elafibranor was well tolerated. Mild adverse events, such as nausea (~10%), headache (8%), diarrhea (6%), fatigue (6%), abdominal pain (9%), vomiting (3%) or rash (4%) were found in the 120-mg elafibranor group. Elafibranor treatment did not induce weight gain or cardiac events, but produced a mild, reversible increase in serum creatinine levels (elafibranor vs. placebo, increase of 4.3±1.2 µmol/L, P<0.001). However, the recent interim analysis from the RESOLVE-IT phase 3 placebo-controlled randomized trial (NCT02704403) showed that elafibranor 120 mg once daily in patients with NASH neither achieved the primary NASH endpoint (i.e., NASH resolution without worsening of fibrosis) nor improved metabolic parameters [52]. As a result, development of this drug was halted.
Saroglitazar
Saroglitazar (ZYH1) is another promising dual PPARα/γ agonist that was designed to have a weaker PPAR-γ effect to reduce the side effects of PPAR-γ agonism, such as weight gain [53]. A meta-analysis involving 318 patients with imaging-defined NAFLD suggested that treatment with saroglitazar may improve serum aminotransferase levels and liver stiffness (by Fibroscan®) in patients with dyslipidemia attributed to diabetes [54]. Recently, in a phase 2 placebo-controlled randomized trial involving 106 obese patients with NAFLD or NASH, who were randomly assigned to receive saroglitazar 1 mg, saroglitazar 2 mg, saroglitazar 4 mg per day or placebo for 16 weeks, the authors found that only saroglitazar 4 mg per day significantly reduced liver fat content (as assessed by magnetic resonance imaging-based proton density fat fraction) and improved serum liver enzymes, insulin resistance, and atherogenic dyslipidaemia [55]. Saroglitazar caused a mean of 1.5 kg weight gain and the drug was well tolerated. The most frequently reported adverse events in the saroglitazar group were diarrhea (~3%), cough (3%), abdominal pain (2%) and bronchitis (1.9%), but they were mild and moderate.
Pioglitazone
Pioglitazone is a well-known insulin sensitizer that improves peripheral insulin sensitivity by activating PPAR-γ, and it is the only TZD currently in use for the treatment of T2DM [45]. Pioglitazone exerts beneficial effects on atherosclerotic processes and the risk of major adverse cardiovascular events [56,57]. Moreover, pioglitazone causes a redistribution of fat from liver and visceral depots to subcutaneous adipose tissue, increases the secretion of adiponectin, and suppresses low-grade inflammation and oxidative stress by activating PPAR-γ. Pioglitazone also induces the expression of multiple genes in hepatocytes, kupffer and stellate cells, thereby promoting a reduction in hepatic inflammation and fibrogenesis [36,58,59].
A small meta-analysis of five phase 2 randomized controlled trials showed that treatment with pioglitazone (at a daily dosage of 30 or 45 mg for a duration up to 24 months) was associated with significant improvements in advanced fibrosis and fibrosis of any stage amongst patients with biopsy-proven NASH, regardless of the presence or absence of T2DM [60]. However, longer randomized controlled trials are needed to confirm the possible beneficial effects of pioglitazone on liver fibrosis and also to test the long-term effects of lower doses of pioglitazone that are associated with fewer side effects. In a placebo-controlled randomized controlled trial including 101 patients with prediabetes or T2DM and biopsy-confirmed NASH, long-term treatment with pioglitazone at the higher dose of 45 mg/day for 72 weeks was associated with an improvement in the individual histological components of NASH [54]. Treatment with pioglitazone was also associated with an improvement in 2 points of NAFLD activity score, and greater NASH resolution without a worsening in fibrosis compared to placebo [58]. Pioglitazone also improved serum liver enzymes, insulin resistance, lipids and proinflammatory biomarkers. However, the wider clinical use of pioglitazone is influenced by its long-term safety, because of moderate weight gain, peripheral fluid retention potentially leading to congestive heart failure (mostly in patients with unrecognized cardiomyopathy), and increased risk of distal bone fractures in post-menopausal women [60,61]. Thus, the current European and American practice guidelines recommend that pioglitazone may be used in adults with biopsy-proven NASH, but patients need to carefully selected before treatment is initiated [17,62].
GLP-1RAs
GLP-1 is an endogenous intestinal hormone that is released by the entero-endocrine L-cells. GLP-1 stimulates pancreatic β-cells to release insulin and inhibits pancreatic α-cells to secrete glucagon [63]. GLP-1RAs reduce food intake, increase glucose uptake in both skeletal muscle and adipose tissue, and reduce hepatic inflammation [64]. Nevertheless, the beneficial effects of GLP-1RAs on both NASH resolution and improvement in fibrosis stage are not fully understood. Because of the lack of GLP-1 receptors in the liver in humans, accumulating evidence suggests that the hepatic effects of GLP-1RAs treatment are most likely due to the reduction of body weight and insulin resistance that lead to subsequent improvements in metabolic dysfunction, lipotoxicity and low-grade inflammation [65-69]. For these reasons, GLP-1RAs are now fast becoming the most favored agents for the treatment of NAFLD, particularly for patients with coexisting obesity or T2DM [70]. Recently, Mantovani et al. [71] undertook a meta-analysis of eleven phase-2 randomized controlled trials (including 936 middle-aged obese or overweight individuals) that used liraglutide (n=6 trials), exenatide (n=3 trials), dulaglutide (n=1 trial) or semaglutide (n=1 trial) to specifically treat NAFLD or NASH, as detected by either imaging techniques or liver biopsy. These authors reported that treatment with GLP-1RAs for a median of 26 weeks was associated with a significant improvement in the absolute percentage of liver fat content on magnetic resonance-based techniques (-3.92%, 95% confidence interval [CI], -6.27% to -1.56%) and serum liver enzyme levels compared to placebo or reference therapy [71]. In the section below, we specifically discuss the results from the only two placebo-controlled randomized controlled trials that used liver biopsy for testing the efficacy of GLP-1RAs (i.e., once-daily subcutaneous semaglutide or liraglutide) for specifically treating NASH in adults with or without T2DM.
Semaglutide
Semaglutide is a long-acting GLP-1RA with more marked metabolic effects than liraglutide, such as reducing body weight, and improving glucose and fatty acid metabolism in the liver [72]. In a multinational phase 2 randomized controlled trial (NCT02970942), 320 obese patients with biopsy-confirmed NASH and fibrosis (F1 to F3 stages) were randomly assigned to the following four treatment arms: 80 patients received subcutaneous semaglutide 0.1 mg/day, 78 patients received semaglutide 0.2 mg/day, 82 patients received semaglutide 0.4 mg/day, and 80 patients received placebo for 72 weeks. The primary study endpoint was the resolution of NASH with no worsening of fibrosis, while the secondary study endpoint was the improvement of at least one fibrosis stage without worsening of NASH. The proportion of patients in whom NASH resolution was achieved with no worsening of fibrosis was 40% in the 0.1 mg group, 36% in the 0.2 mg group, 59% in the 0.4 mg group, and 17% in the placebo group (P<0.001 for semaglutide 0.4 mg vs. placebo); improvement in fibrosis stage occurred in 43% of the patients in the 0.4 mg group and in 33% of the patients in the placebo group (P=0.480 for semaglutide 0.4 mg vs. placebo). Treatment with semaglutide also resulted in dose-dependent reductions of body weight, serum liver enzymes and metabolic parameters [73]. The most common adverse events of semaglutide are gastrointestinal side effects, such as nausea (~42%), constipation (22%), decreased appetite (23%), diarrhea (20%), vomiting (15%) and abdominal pain (7%) in the 0.4-mg semaglutide group [73]. If these promising results are confirmed by ongoing large phase-3 randomized controlled trials, semaglutide will become an important treatment option for patients with NAFLD or NASH, who benefit from weight loss.
Liraglutide
Liraglutide is another safe and well-tolerated GLP-1RA drug that may benefit NASH [74]. In the small phase 2b LEAN trial (NCT01237119) that involved 52 UK obese patients with biopsy-proven NASH, treatment with subcutaneous liraglutide 1.8 mg/day for 48 weeks resulted in a higher proportion of patients with NASH resolution than placebo. In fact, 39% of patients treated with liraglutide achieved a histologic resolution of NASH vs. 9% in the placebo group (P=0.019), and only 9% of patients in the liraglutide group had progression of fibrosis vs. 36% patients in the placebo group (P=0.04) [75]. In a meta-analysis involving 1,557 patients with T2DM, treatment with liraglutide also improved serum liver enzymes and reduced the risk of major adverse cardiovascular events [76]. Gastrointestinal side effects, e.g., nausea (~46%), diarrhea (38%), abdominal pain (31%), constipation (27%), vomiting (19%) and dyspepsia (15%), are the most common side effects of liraglutide [75].
DPP-4 inhibitors
DPP-4 inhibitors are widely used as oral glucose-lowering drugs for the treatment of T2DM. DPP-4 inhibitors prolong the biologic life of incretins and promote pancreatic insulin production [77,78]. These drugs have a good safety profile in the absence of any gastrointestinal disorders. To date, however, there is no data available testing the efficacy of DPP-4 inhibitors on liver histology among patients with biopsy-proven NAFLD or NASH. It has been observed that the levels of serum DPP-4 activity were increased in patients with more severe NAFLD, suggesting that lowering DPP-4 activity could be beneficial inNASH [79].
Sitagliptin
Sitagliptin has been widely used for over 10 years and has a well-characterized safety and tolerability profile [80]. A small open-label controlled trial showed that sitagliptin improved histologic NAFLD activity score in patients with NAFLD, regardless of the diabetes status [81]. A 26-week multicenter trial in China (NCT02147925) showed that combined with metformin, sitagliptin reduced body weight, hepatic fat content and visceral adipose tissue in addition to improving glycaemic control in patients with T2DM and NAFLD [82]. A small study involving 41 T2DM patients (20 men and 21 women) also showed that DPP-4-therapy for 6 months led to a significant decrease in body weight and improvements in hepatic and myocardial lipid contents (as assessed by magnetic resonance-based techniques) only in women [83]. However, two small clinical trials using sitagliptin failed to show any beneficial effects on liver steatosis or fibrosis in patients with NAFLD [77]. One of these two small clinical trials involved 50 patients with NAFLD who were randomly assigned to receive sitagliptin 100 mg/day or placebo. After 24 weeks, there were no significant improvements neither in liver steatosis or fibrosis nor in serum liver enzymes and lipid profile between the two treatment arms. However, it might due to that the period of treatment is too short [84,85]. Sitagliptin is usually well tolerated, and there are no significant adverse events documented [83,85]. However, there is not sufficient evidence to advocate use of sitagliptin as a treatment for NAFLD.
Vildagliptin
Vildagliptin is another oral incretin-based DPP-4 inhibitor, which promotes pancreatic insulin production, inhibits glucagon secretion, delays gastric emptying, reduces appetite and has a low risk of weight gain and hypoglycaemia [86]. In a small phase 2 randomized controlled trial involving 58 dyslipidemic patients with NAFLD, a 12-week treatment with vildagliptin led to improvements in hepatic fat content on ultrasonography as well as plasma lipid profile and liver enzymes [87].
SGLT-2 inhibitors
SGLT-2 inhibitors are a newer class of oral glucose-lowering agents that act by decreasing glucose reabsorption in the renal proximal tubule. Moreover, SGLT2 inhibitors induce weight loss, reduce the risk of major adverse cardiovascular events (including hospitalization for heart failure) and have beneficial effects on renal function [88]. Many studies also showed that SGLT-2 inhibitors reduce hyperglycemia, and improve proinflammatory biomarkers, thus these drugs are strongly recommended in people with T2DM and pre-existing cardiovascular disease, or who are at high cardiovascular risk [89,90]. Recently, Mantovani et al. [91] performed an updated meta-analysis of twelve randomized clinical trials testing the efficacy of dapagliflozin (n=6 trials), empagliflozin (n=3 trials), ipragliflozin (n=2 trials) or canagliflozin (n=1 trial) to specifically treat NAFLD (as assessed by magnetic resonance-based techniques) for a median period of 24 weeks with aggregate data on 850 individuals with NAFLD (90% with T2DM). Compared to placebo or reference therapy, treatment with SGLT-2 inhibitors significantly decreased serum liver enzyme levels, and improved the absolute percentage of liver fat content on magnetic resonance-based techniques (-2.05%; 95% CI, -2.61% to -1.48%). More recently, Takahashi et al. [92] conducted an open-label randomized controlled trial that aimed to examine the effect of ipragliflozin on hepatic pathology in 50 patients with T2DM and biopsy-proven NAFLD. These authors reported that patients treated with ipragliflozin (50 mg daily, n=24) for 72 weeks had better hepatic histology outcomes, including the severity of liver fibrosis and ballooning, compared to patients (n=26) who performed lifestyle modifications and/or took glucose-lowering drugs, with the exception of SGLT2 inhibitors, pioglitazone, or GLP-1RAs [92]. To date, however, no robust data from sufficiently large randomized controlled trials with liver histological endpoints are available to comment on the long-term efficacy of SGLT2 inhibitors as a treatment for NASH.
ONGOING RANDOMIZED CLINICAL TRIALS
In Table 2 we have listed the most relevant ongoing phase 2 and phase 3 placebo-controlled randomized clinical trials testing the efficacy of newer glucose-lowering agents for specifically treating NAFLD or NASH in adults with or without established T2DM.
SUMMARY AND CONCLUSIONS
To date, there is still no licensed treatment for NAFLD or NASH. However, there is now increasing evidence of efficacy in adults with biopsy-confirmed NASH with two glucose-lowering treatments, namely pioglitazone and GLP-1RA agents (e.g., semaglutide and liraglutide). Although long-term treatment with pioglitazone or GLP-1RAs is associated with some side effects, both classes of drugs also have proven benefits to reduce the risk of major adverse cardiovascular events. These additional benefits are potentially important and clinicians should consider these extra-hepatic benefits of treatment, in making an informed decision to use these drugs in patients with T2DM and NAFLD (or NASH). For those patients who do not have T2DM but have NAFLD (or NASH), further research is needed, but current evidence suggests that PPAR agonists (mostly pioglitazone and lanifibranor) and GLP-1RAs are also beneficial in this group of patients. That said, if the promising results with lanifibranor and GLP-1RAs are confirmed in larger placebo-controlled randomized trials, it is reasonable to suggest that PPAR agonists, GLP-1RAs, and possibly also SGLT2 inhibitors (singularly or in combination) are likely to become important treatment options for patients with NAFLD or NASH, regardless of the presence or absence of T2DM.
Notes
Authors’ contributions
Conception and design: L Miao, MH Zheng; Collection and assembly of data: L Miao, J Xu; Manuscript writing, intellectual input, critical evaluation and proofreading: all authors; Final approval of manuscript: all authors.
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 82070588), High Level Creative Talents from the Department of Public Health in Zhejiang Province (No. S2032102600032), Project of New Century 551 Talent Nurturing in Wenzhou. GT is supported in part by grants from the University School of Medicine of Verona, Verona, Italy. CDB is supported in part by the Southampton NIHR Biomedical Research Centre (IS-BRC-20004), UK. This work is a part of the PERSONS study.
Abbreviations
CI
confidence interval
DPP-4
dipeptidyl peptidase-4
GLP-1RA
glucagon-like peptide-1 receptor agonist
MAFLD
metabolic dysfunction-associated fatty liver disease
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
PPAR
peroxisome proliferator-activated receptor
SAF
Steatosis
SGLT-2
sodium-glucose cotransporter-2
T2DM
type 2 diabetes mellitus
TZD
thiazolidinedione

