Effects of interleukin-17 inhibitors on hepatic fibrosis index in patients with psoriasis and metabolic dysfunction-associated fatty liver disease: Directed acyclic graphs
Article information
Dear Editor,
Recently, metabolic dysfunction-associated fatty liver disease (MAFLD) has been proposed [1]. The feature of MAFLD is the inclusion of metabolic dysfunctions and is independent of alcohol intake [2]. Psoriasis is a dermatosis associated with metabolic syndrome and alcoholic intake [3]. However, no information is available on the impact of MAFLD on the severity of psoriasis.
Hepatic fibrosis is a prognostic factor in patients with MAFLD [1,2] and interleukin-17 (IL-17) is involved in the pathogenesis of hepatic fibrosis [4]. IL-17 causes neutrophil infiltration, insulin resistance, and subsequent steatohepatitis [5]. IL-17 is also involved in the pathogenesis of psoriasis, and IL-17 inhibitor (IL-17i) is an approved agent for psoriasis with high efficacy [3]. Herein, we investigated the impact of MAFLD in patients with psoriasis. We also investigated the effect of IL-17i on hepatic fibrosis and its contributing factors.
We enrolled 65 consecutive patients with psoriasis treated with IL-17i in this retrospective study approved by the Institutional Review Board of Kurume University School of Medicine (ID 21236). Data were collected before and 6 months after IL-17i treatment (secukinumab or Ixekizumab). The severity of psoriasis was evaluated by psoriasis area and severity index (PASI) [3]. MAFLD was diagnosed as previously described [1].
The participant characteristics are summarized in Supplementary Table 1. The prevalence of MAFLD was in 82.6% of enrolled patients. A high non-alcoholic fatty liver disease (NAFLD) fibrosis score (>-1.455), high fibrosis-4 (FIB-4) index (>1.3), and low platelet count (<20.0×104/µL) were observed in 24.6%, 21.1%, and 16.9% of patients (Supplementary Fig. 1). These prevalences are relatively higher than the previous reports using NAFLD criteria [6,7]. A possible reason is that we employed the MAFLD definition and included patients with metabolic dysfunctions and >20 g/day of alcohol intake, which was excluded in the previous studies [6,7].
We employed a decision tree analysis to reveal a series of classification factors for the severity of psoriasis. Sex was identified as the most important classifier for the PASI level (Fig. 1A). In men, the second classifier was MAFLD. The median PASI was 11.5 in patients with MAFLD, while the PASI was 6.3 in patients with non-MAFLD. Immune dysregulation mediated by the IL-17 pathway is the well-known pathogenesis of psoriasis [8]. The IL-17 effectors cause keratinocyte hyperproliferation and inflammation [8]. Whereas, overnutrition induces inflammation via T helper 17 cells and IL-17, resulting in neutrophil infiltration to adipose tissue, insulin resistance, and subsequent non-alcoholic steatohepatitis (NASH) in mice [5]. The hepatic expression of T helper 17 cell-related genes is up-regulated in patients with NASH and IL-17 facilitates the transition from simple steatosis to NASH [9]. Thus, IL-17 may be a key molecule linking MAFLD and exacerbation of psoriasis.
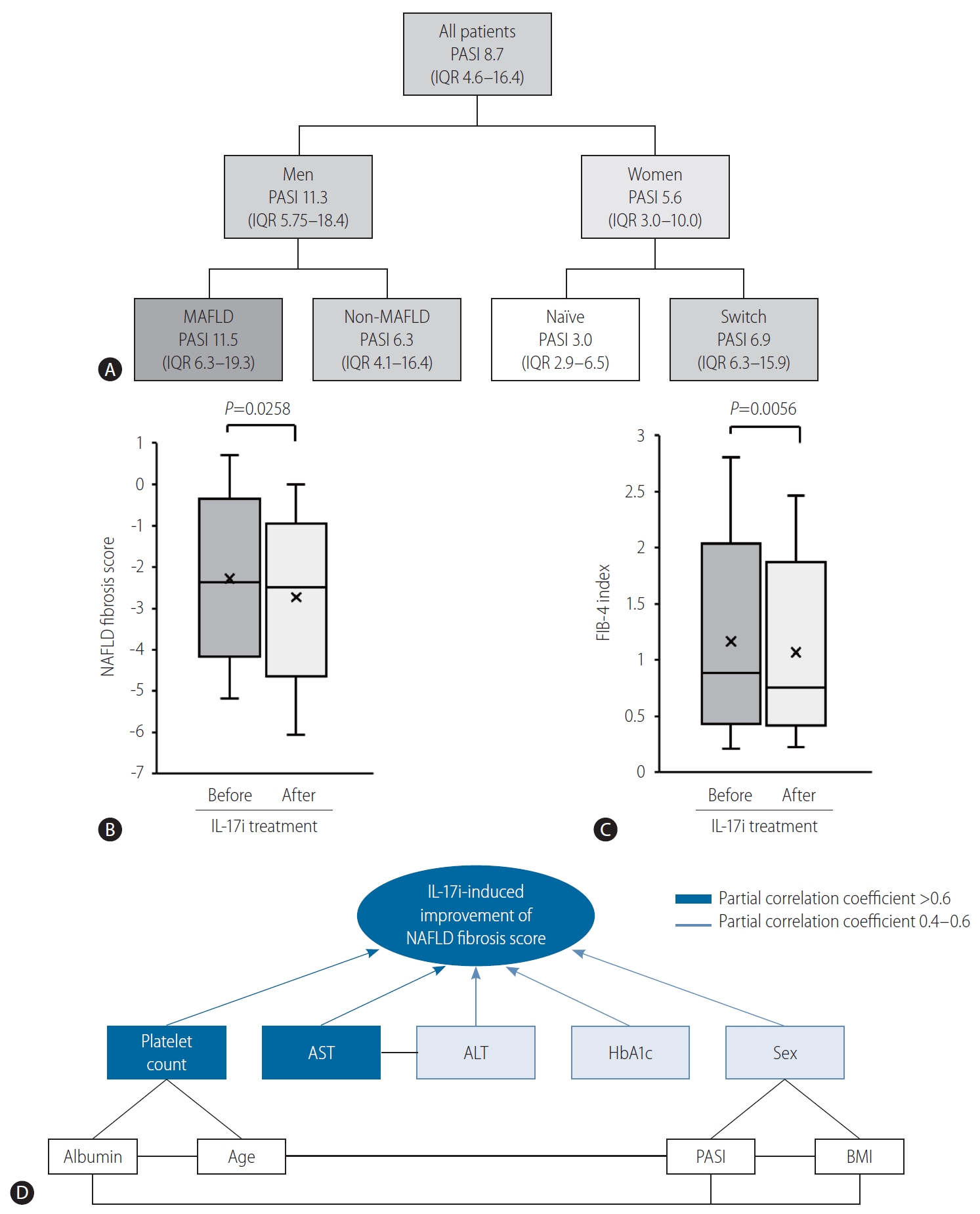
(A) A decision tree analysis for PASI levels. The classifier is indicated by the underline. (B) Changes in NAFLD fibrosis score six months after IL-17i treatment in patients with MAFLD. (C) Changes in FIB-4 index 6 months after IL-17i treatment in patients with MAFLD. (D) DAGs for the IL-17i-induced improvement of NAFLD fibrosis score. PASI, psoriasis area and severity index; IQR, interquartile range; MAFLD, metabolic dysfunction-associated fatty liver disease; NAFLD, non-alcoholic fatty liver disease; IL-17i, interleukin-17 inhibitor; FIB-4, fibrosis-4; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HbA1c, hemoglobin A1c; BMI, body mass index.
Changes in biochemical parameters after IL-17i treatment are summarized in Supplementary Table 2. NAFLD fibrosis score was significantly decreased 6 months after IL-17i treatment (Fig. 1B). A significant decrease was also observed in the FIB-4 index (Fig. 1C). IL-17 directly induces the production of type I collagen in hepatic stellate cells by up-regulation of transforming growth factor β [4,10]. In addition, suppression of IL-17 inhibits activation of hepatic stellate cells and collagen production in vitro [11]. Moreover, treatment with anti-IL-17 antibody improved hepatic fibrosis in a NASH mice model [12]. A recent pilot study showed that IL-17i improved hepatic fibrosis evaluated by transient elastography in 10 patients with psoriasis [13]. Thus, accumulating evidence, along with our results suggest that IL-17i may be beneficial for the improvement of hepatic fibrosis in patients with psoriasis and MAFLD.
Differences in baseline characteristics between the patients with improvement and with non-improvement of NAFLD fibrosis score after IL-17i treatment are summarized in Supplementary Table 3. We employed directed acyclic graphs to investigate causal relationships between the IL-17i-induced improvement of NAFLD fibrosis score and its associated factors. We revealed that up-regulation of platelet count and down-regulation of aspartate aminotransferase level were major contributing factors for the improvement of NAFLD fibrosis score (Fig. 1D). IL-17 signaling promotes apoptosis of human megakaryocyte cells, and neutralization of IL-17 up-regulates anti-apoptotic Bcl-2 protein in megakaryocyte cells [14]. Moreover, IL-17 exacerbates hepatic inflammation through regulatory T cell-mediated responses in a mouse model of NAFLD [9]. Furthermore, anti-IL-17 antibody or knock-out of IL-17 gene ameliorates hepatic inflammation in mouse models of NASH [5,12,15]. These basic studies support our results.
There are several limitations to this study. First, this is a two-center retrospective study with small sample size. Second, we did not evaluate hepatic fibrosis by imaging modalities. Thus, further study should be designed as a multi-center prospective study with imaging modalities for hepatic fibrosis.
In conclusion, MAFLD was highly prevalent in patients with psoriasis and was associated with the severity of psoriasis in men. Moreover, IL-17i, a biologic agent for psoriasis, improved the NAFLD fibrosis score. This improvement was independent of the amelioration of psoriasis and mainly caused by alterations in platelet count and aspartate aminotransferase level. Thus, IL-17i may improve hepatic fibrosis through the regulation of platelet count and hepatic inflammation.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (C) JP20K08395, Grant-in-Aid for Young Scientists (B) JP19K17446, and by the Research Program on Hepatitis from Japan Agency for Medical Research and Development, AMED under 21fk0210094.
Notes
Authors’ contributions
Saori Takamura, Yuichi Teraki, and Takumi Kawaguchi participated in study conception and design. Saori Takamura, Yuichi Teraki, Eri Katayama, and Takekuni Nakama participated in acquisition of data. Takumi Kawaguchi, Machiko Kawaguchi, Dan Nakano, and Tsubasa Tsutsumi participated in analysis. Saori Takamura, Yuichi Teraki, Takumi Kawaguchi, Sumiko Nagoshi, Takekuni Nakama, and Takuji Torimura participated in interpretation of data. Saori Takamura, Yuichi Teraki, Takumi Kawaguchi, Sumiko Nagoshi, Takekuni Nakama, and Takuji Torimura drafting of manuscript. Yuichi Teraki, Sumiko Nagoshi, Takekuni Nakama, and Takuji Torimura participated in critical revision. All authors contributed to revisions and approved the final manuscript.
Conflicts of Interest
The authors have no conflicts to disclose.
Abbreviations
FIB-4
fibrosis-4
IL-17
interleukin-17
IL-17i
IL-17 inhibitor
MAFLD
metabolic dysfunction-associated fatty liver disease
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
PASI
psoriasis area and severity index
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Patients’ characteristics of all subjects
Changes in severity of psoriasis and biochemical parameters 6 months after IL-17i treatment in patients with MAFLD
Differences in baseline characteristics between the patients with improvement and with non-improvement of NAFLD fibrosis score 6 months after IL-17i treatment in patients with MAFLD
The prevalence of MAFLD and high hepatic fibrosis indexes. (A) MAFLD, (B) NAFLD fibrosis score, (C) FIB-4 index, and (D) platelet count. Normal or high NAFLD fibrosis score was classified based on the cut-off value of -1.455. Normal or high FIB-4 index was classified based on the cut-off value of 1.3. Normal or low platelet count was classified based on the cut-off value of 20.0×104/µL. NAFLD, non-alcoholic fatty liver disease; MAFLD, metabolic dysfunction-associated fatty liver disease; FIB-4, fibrosis-4.
