Application of transient elastography in nonalcoholic fatty liver disease
Article information
Abstract
Nonalcoholic fatty liver disease (NAFLD) is currently the most common chronic liver disease worldwide. Although it has become one of the leading causes of cirrhosis and hepatocellular carcinoma in the Western world, the proportion of NAFLD patients developing these complications is rather small. Therefore, current guidelines recommend non-invasive tests for the initial assessment of NAFLD. Among the available non-invasive tests, transient elastography by FibroScan® (Echosens, Paris, France) is commonly used by hepatologists in Europe and Asia, and the machine has been introduced to the United States in 2013 with rapid adoption. Transient elastography measures liver stiffness and the controlled attenuation parameter simultaneously and can serve as a one-stop examination for both liver steatosis and fibrosis. Liver stiffness measurement also correlates with clinical outcomes and can be used to select patients for varices screening. Although obesity is a common reason for measurement failures, the development of the XL probe allows successful measurements in the majority of obese patients. This article reviews the performance and limitations of transient elastography in NAFLD and highlights its clinical applications. We also discuss the reliability criteria for transient elastography examination and factors associated with false-positive liver stiffness measurements.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is currently the most common chronic liver disease worldwide [1]. In the United States, nonalcoholic steatohepatitis (NASH), the active form of NAFLD with faster fibrosis progression, has already become one of the leading causes of cirrhosis and hepatocellular carcinoma [2]. An updated systematic review and meta-analysis also shows that the prevalence of NAFLD in Asia has increased from 25% between 1999 and 2005 to 34% between 2012 and 2017 [3]. However, because of the large number of NAFLD patients and that only a minority of patients would eventually develop liver-related morbidity and mortality, non-invasive tests are preferred as initial assessment.
Among the available non-invasive tests, vibration-controlled transient elastography (FibroScan®, Echosens, Paris, France) is commonly used by hepatologists in Europe and Asia [4]. In 2013, the machine was approved by the United States Food and Drug Administration and has rapidly been introduced to different American centers since then. The latest models of transient elastography have different probes (S, M, and XL) to cater for patients with different body build. They also measure both the controlled attenuation parameter (CAP) for hepatic steatosis and liver stiffness for liver fibrosis. In this article, we review the literature on the performance of CAP and liver stiffness measurement (LSM). We discuss the use of M and XL probes, and the reliability criteria of transient elastography examination. Because some but not all studies suggest that hepatic steatosis may confound LSM, we specifically review this issue and highlight other confounding factors.
LIVER HISTOLOGY
NAFLD is an umbrella term covering the spectrum of disease ranging from nonalcoholic fatty liver (NAFL) to NASH [5]. NAFL is defined as the presence of hepatic steatosis with no hepatocellular injury [6]. NASH is defined as the presence of hepatic steatosis and inflammation with hepatocyte injury with or without fibrosis, which is the more progressive form of NAFLD [7]. A subset of patients with NAFLD develop progressive fibrosis, with risk of progression to cirrhosis and hepatocellular carcinoma [8]. Key histological features of NAFLD include steatosis, lobular inflammation, portal inflammation, hepatocyte ballooning and fibrosis [9]. Among the histological features, fibrosis stage has the strongest prognostic significance. The NASH Clinical Research Network system adopts a 5-point fibrosis staging system (0, no fibrosis; 1, perisinusoidal or portal/periportal only; 2, perisinusoidal and periportal; 3, bridging fibrosis; 4, cirrhosis) [10]. Fibrosis stage is the most important histological predictor of all-cause and liver related mortality [11,12]. A systematic review and meta-analysis reported that the risk of liver-related mortality increased with fibrosis stage [13]. A number of issues limit the wide application of liver biopsy for the assessment of NAFLD. Firstly, liver biopsy is an invasive procedure associated with pain and discomfort, and even a small risk of complications such as bleeding [4]. Secondly, liver biopsy is not widely acceptable for its costs and availability and it is impractical to do it repeatedly to assess disease progression [14]. Thirdly, sampling variability is common because a biopsy specimen only represents only 1/50,000 of the liver volume, which easily causes misdiagnosis [15]. Finally, interobserver variation also heavily influences the assessment of NASH, especially for necroinflammation activity [10]. Therefore, liver biopsy is not a true gold standard for the evaluation of NAFLD. There is an urgent need to find non-invasive ways to assess patients with NAFLD.
CONTROLLED ATTENUATION PARAMETER (CAP)
Mechanism
The latest model of transient elastography measures CAP and liver stiffness simultaneously [16]. The former reflects the degree of hepatic steatosis. The typical features of fatty liver on abdominal ultrasonography include bright liver echotexture, deep attenuation of ultrasound signal and vascular blunting [5]. The latter two features are because of the faster attenuation of ultrasound wave amplitude in a steatotic liver. CAP takes advantage of this physical property and estimates the ultrasound attenuation at the central frequency of transient elastography, while assuming a homogeneous fat distribution and an adequate penetration [17].
Performance
Although abdominal ultrasonography is often the first-line investigation for the diagnosis of NAFLD, it is operator-dependent and is insensitive to mild hepatic steatosis [4]. Typically, hepatic steatosis has to involve more than 30% of hepatocytes before ultrasonography can reliably detect fatty liver [18]. Table 1 summarizes studies comparing the performance of CAP against liver histology for the detection of various steatosis grades [19-31]. Overall, the areas under receiver-operating characteristics curves (AUROC) are 81–84% for ≥S1 (steatosis in at least 5–10% of hepatocytes), 85–88% for ≥S2 (33%), and 86–91% for S3 (66%) steatosis. The reported sensitivities for ≥S1, ≥S2 and S3 are 60–75%, 69-84% and 77–96%, respectively. The corresponding specificities are 76–90%, 75–88% and 72–82%, respectively. A patient-level meta-analysis of nineteen studies involving 2,735 patients shows similar findings, with a pooled sensitivities and specificities of 69% and 82% for ≥S1, 77% and 81% for ≥S2, and 88% and 78% for S3 [32]. The optimal cutoffs of CAP are 248 dB/m for S1, 268 dB/m for S2 and 280 dB/m for S3. It should be noted that some studies used patients with other liver diseases as controls without hepatic steatosis. Since such patients underwent liver biopsies with other indications, they do not represent a healthy population of subjects. This may have affected the derivation of optimal cutoffs and the evaluation of test performance. Furthermore, the test evaluation is affected by patient composition. Studies based on more obese NAFLD cohorts in Europe and North America usually recommend higher cutoff values for each steatosis grade [30,31].
Magnetic resonance imaging (MRI) such as estimated proton density fat fraction (MRI-PDFF) and proton-magnetic resonance spectroscopy is also a leading non-invasive test for steatosis quantification with high sensitivity and specificity [33]. MRI is not affected by obesity and has higher success rate of examination than transient elastography [27]. Recently, Caussy and colleagues compared CAP and MRI-PDFF in 119 individuals with and without NAFLD [34]. The AUROC of CAP to detect MRI-PDFF ≥5% was reasonable at 0.80, whereas that for MRI-PDFF ≥10% was 0.87. The optimal CAP cutoffs for these two MRI-PDFF thresholds were 288 dB/m and 306 dB/m.
While most of the validation studies are cross-sectional in nature, it is important to remember that patients often require serial examinations for disease progression and treatment response. In that regard, MRI-PDFF is precise and can detect small changes in hepatic steatosis over time [35]. In contrast, although CAP is reproducible on repeated testing [36], further longitudinal studies should clarify its performance as a monitoring tool.
Clinical applications
Although MRI-PDFF has superior performance to CAP, the former is limited by cost and availability [34]. In clinical practice, CAP is a reasonable test for the diagnosis of NAFLD, especially as abdominal ultrasonography may be falsely negative when <30% of hepatocytes are steatotic [37]. Studies have shown that CAP has strong association with metabolic syndrome, body mass index (BMI), and chronic hepatitis C [19,32,38]. CAP is therefore an important and non-invasive method for screening fatty liver in the general population or high-risk population such as patients with type 2 diabetes, obesity and chronic liver diseases (Table 2) [39,40].
LIVER STIFFNESS MEASUREMENT (LSM)
Mechanism
During LSM by transient elastography, vibrations of mild amplitude and low frequency (50 Hz) are transmitted by the transducer, inducing an elastic shear wave that propagates through the underlying tissues [41]. Pulse-echo ultrasound acquisition is used to follow the propagation of the shear wave and to measure its velocity, which is directly related to tissue stiffness: the stiffer the tissue, the faster the shear wave propagates [16]. LSM values range from 1.5 to 75 kPa; lower values indicate a more elastic liver [42]. Transient elastography measures liver stiffness in a volume at least 100 times bigger than a biopsy sample, and is therefore far more representative and reliable [43].
Performance
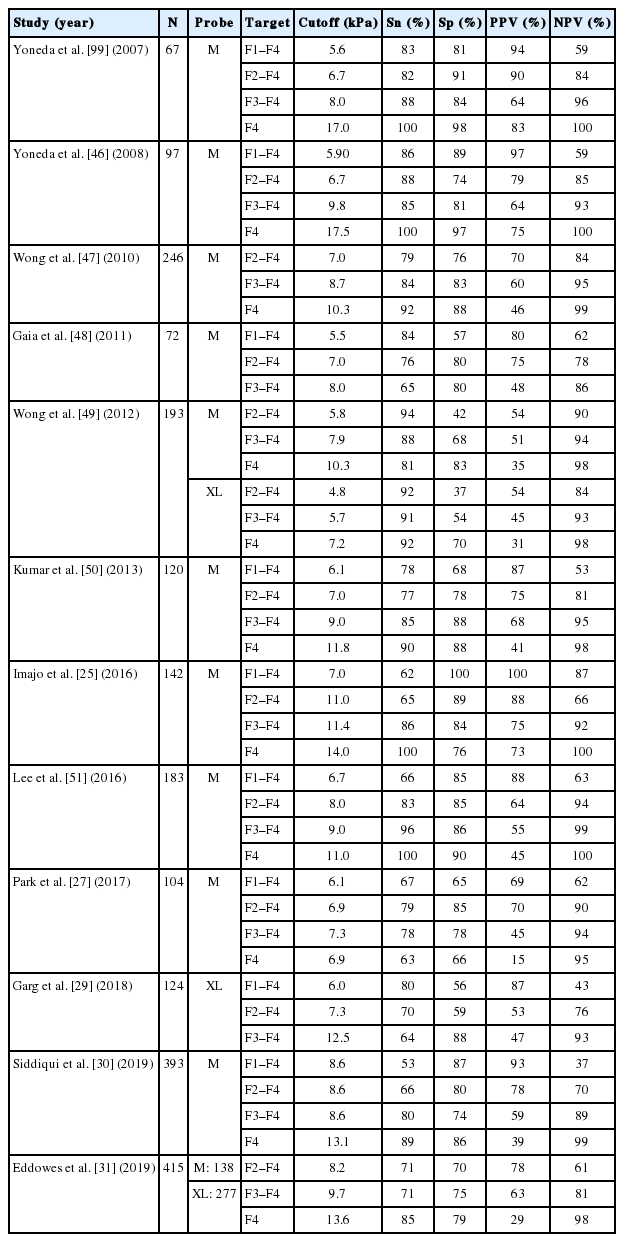
Transient elastography is painless, rapid and easy to perform at the bedside or in the clinic. It allows rapid and non-invasive estimation of hepatic fibrosis in patients with various chronic liver diseases including chronic hepatitis C [44], chronic hepatitis B [45], and NAFLD [46]. Table 3 shows studies on the performance of LSM in NAFLD patients compared with liver biopsy [25,27,29-31,47-51]. Overall, the AUROC of LSM for stages F1, F2, F3, and F4 were 0.82, 0.85, 0.94, and 0.96, respectively. For F2–4 fibrosis, the LSM cut-off values range from 6.2 to 11 kPa, with 62–90% sensitivity and 74–100% specificity. For F3–4 fibrosis, the LSM cut-off values range from 8 to 12 kPa, with 84–100% sensitivity and 83–97% specificity. For F4, the LSM cut-off values range from 9.5 to 20 kPa, with 90–100% sensitivity and 75.9–98.4% specificity.
In a meta-analysis of nine studies [52], the estimates for sensitivity were 87% (95% confidence interval [CI], 84–90%), specificity 91% (95% CI, 89–92%), positive likelihood ratio 11.7 (95% CI, 7.9–17.1), and negative likelihood ratio 0.14 (95% CI, 0.10–0.20). The optimal cutoff varies across studies as it is a tradeoff between sensitivity and specificity and may be influenced by the underlying liver disease.
Magnetic resonance elastography (MRE) is a MRI-based method for quantitatively imaging tissue stiffness. Multiple studies have shown that MRE can be a useful method for the diagnosis of liver fibrosis in patients with NAFLD, even in the early stages. Imajo and colleagues performed a cross-sectional study comparing transient elastography and MRE in 142 Japanese patients with biopsyproven NAFLD [25]. The AUROC curve in diagnosing liver fibrosis stage 1, 2, 3, and 4 using MRE and transient elastography were 0.80 vs. 0.78, 0.89 vs. 0.82, 0.89 vs. 0.88, and 0.97 vs. 0.92, respectively. The reported sensitivities for F1–4, F2–4, F3–4 and F4 fibrosis were 75% vs. 61.7%, 87.3% vs. 65.2%, 74.5% vs. 85.7% and 90.9% vs. 100%, respectively. The corresponding specificities were 85.7% vs. 100%, 85% vs. 88.7%, 86.9% vs. 83.8% and 94.5% vs. 75.9%, respectively. The findings were confirmed in another study with head-to-head comparison of the two techniques in the United States [27]. The results indicate that MRE has higher diagnostic accuracy in the assessment of liver fibrosis than transient elastography, though the absolute difference is marginal.
LSM by transient elastography is highly reproducible. In the study by Fraquelli and colleagues [53], 800 transient elastography examinations were performed in 200 patients with chronic liver diseases and the overall interobserver agreement intraclass correlation coefficient (ICC) was 0.98 (95% CI, 0.977 to 0.987). However, increased body mass index (25 kg/m2), steatosis, and fibrosis stage <2 were associated with reduced ICC.
Predicting liver related complications
Transient elastography not only allows early identification of patients with fibrosis and cirrhosis but also plays an important part in predicting complications of compensated advanced chronic liver disease (cACLD), such as gastroesophageal varices, hepatocellular carcinoma and liver-related deaths. Variceal hemorrhage is a common and severe complication of cACLD. Screening for the presence of esophageal varices (EV) with esophagogastroduodenoscopy (EGD; the gold standard) in cirrhotic patients is recommended by current guidelines, but EGD is costly and inconvenient. Many studies have shown that transient elastography has potential value in the prediction of EV [54-56]. The Baveno VI consensus states that patients with LSM <20–25 kPa and a normal platelet count of ≥150×109/L are unlikely to harbor varices needing treatment and may be spared from endoscopy [57]. This notion has since been validated in different settings. In a large multicenter cohort of patients with NASH-related cirrhosis, Petta and colleagues demonstrated the role of probe-specific LSM and platelet count to detect varices needing treatment [58]. The study also suggests the possibility of loosening the criteria (LSM 30 kPa for M probe and 25 kPa for XL probe; platelet count 110×109/L) to reduce the number of patients requiring endoscopy further without jeopardizing the false-negative rate (Fig. 1).
In addition to EV, one of the most important complications of liver fibrosis progression is the development of hepatocellular carcinoma. Several studies have proposed that transient elastography can be used to assess the risks of development of hepatocellular carcinoma, based on the significant correlation between the risk of hepatocellular carcinoma development and the degree of liver fibrosis [59-61]. A recent systematic review and meta-analysis performed by Singh and colleagues also support these findings [62]. Furthermore, recent studies have shown an association between LSM and survival. Among 2,052 patients with chronic liver diseases, Pang and colleagues reported that LSM by transient elastography had excellent accuracy in predicting the risk of death in patients with chronic liver diseases [63]. On the other hand, CAP does not appear to predict liver-related outcomes [64]. This is in line with liver biopsy studies showing that steatosis is not as important a prognostic marker as the other histological features [65].
Clinical applications
Above all, transient elastography can be performed to estimate severity of liver fibrosis in NAFLD patients at both the primary care and specialist settings. A recent meta-analysis included nine studies consisting of 1,047 NAFLD patients suggests that transient elastography is excellent in diagnosing F3–4 (85% sensitivity, 82% specificity) and F4 fibrosis (92% sensitivity, 92% specificity) and has moderate accuracy for F2–4 fibrosis (79% sensitivity, 75% specificity) [66]. Secondly, transient elastography can contribute to select patients for clinical trials or pharmacological treatment. transient elastography not only has good accuracy and high reproducibility of liver fibrosis, but also has the advantage of being quick, non-invasive, easy to learn and well tolerated by patients, which makes it widely utilize in scientific research [67,68]. Thirdly, transient elastography can be used to screen for liver fibrosis in the general population of high-risk individuals (e.g., type 2 diabetes and obesity). The overall impact of obesity, type 2 diabetes and other metabolic risk factors on liver fibrosis are greatly underestimated by current practice. In a population-based study among individuals over 45 years using transient elastography showed that both BMI >30 kg/m2 and type 2 diabetes were significantly associated with liver stiffness ≥8 kPa [69]. In another study of 1,918 patients with type 2 diabetes, 72.8% had fatty liver and 17.7% had high liver stiffness suggestive of advanced fibrosis, highlighting the importance of case finding or even screening in this high risk population [39]. In patients with NASH-related cirrhosis, LSM is a useful tool to predict varices, hepatocellular carcinoma and liverrelated death. The Baveno VI criteria and its modifications are good starting points to select patients for endoscopic screening [70]. Last but not least, some studies indicate that transient elastography can be used to monitor fibrosis changes after treatment, though this should be confirmed by further studies using paired liver biopsies [71,72].
M AND XL PROBES
One of the biggest challenge of transient elastography examination is the lower success rate in obese patients [73]. This is particularly relevant for NAFLD because of its close association with obesity [74]. To cater for this limitation, the manufacturer of transient elastography has produced different probes to cater for patients with different body build. While the M probe is for average adults, the S probe is for children and adolescents and the XL probe is for obese patients. By using a lower frequency (2.5 MHz instead of 3.5 MHz for the M probe), the XL probe measures CAP and liver stiffness at a greater depth (35–75 vs. 25–65 mm) [75]. Even in the obese population, the XL probe allows successful measurements in the majority of cases [49,76,77].
Because ultrasound-based transient elastography measures the Young’s modulus and is expected to be affected by ultrasound frequency [41], prospective studies indeed confirmed that the XL probe would yield a lower liver stiffness value than the M probe when applied on the same patient [49,76]. Nonetheless, since high body mass index also leads to higher liver stiffness values (see section on confounding factors), the effects of obesity and XL probe on LSM tend to cancel each other. When we applied the M probe in patients with body mass index <30 kg/m2 and XL probe in those ≥30 kg/m2, the median liver stiffness values by both probes were nearly identical at each fibrosis stage, suggesting that one may adopt the same interpretation when the right probe is used for the right patient (Fig. 2) [78]. In our hands, the same CAP cutoffs can also be used for the two probes with similar accuracy [28]. The latest model of transient elastography has an automated probe selection tool that recommends the use of the M or XL probes based on the skin-to-liver capsule distance. When the probe selection tool is followed, again it does not appear that cutoff adjustments are required for the two probes [31].
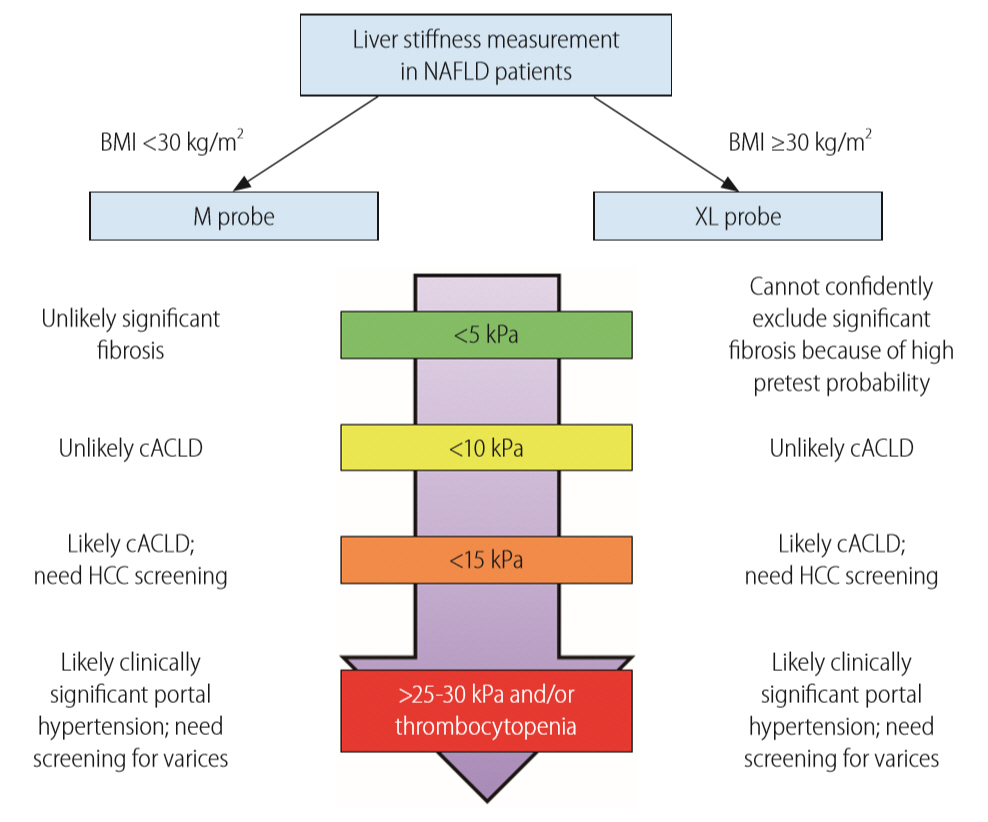
Unified interpretation of liver stiffness measurement by M and XL probes in NAFLD patients. Reproduced from Wong et al. [78] with permission from BMJ Publishing Group Ltd. NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; cACLD, compensated advanced chronic liver disease; HCC, hepatocellular carcinoma.
RELIABILITY CRITERIA
During a transient elastography examination, an operator typically obtains ten measurements. The median values of CAP and liver stiffness reflect the degree of hepatic steatosis and fibrosis, respectively, whereas the interquartile range (IQR) of the ten measurements represents the variability of measurements. Highly variable measurements suggest a difficult examination, suboptimal technique or heterogeneous pathology in the liver parenchyma. According to the original manufacturer recommendations, a reliable LSM is defined as obtaining ten valid measurements, a success rate (number of valid acquisitions divided by the number of attempts) >60%, and an interquartile range-to-median ratio (IQR/M) of ≤0.3. However, subsequent studies have not found success rate to be a determinant of reliable examination [47,79].
In a study of 1,165 French patients with chronic liver disease (798 had chronic hepatitis C), Boursier and colleagues proposed new reliability criteria based on both IQR/M and the median liver stiffness values [80]. In essence, poorly reliable LSM is defined as IQR/M >0.30 and liver stiffness ≥7.1 kPa for F2–3 fibrosis and ≥12.5 kPa for F4 fibrosis. Because LSM has a high negative predictive value but a modest positive predictive value [81], it is reasonable to consider a patient with a liver stiffness of 7.1 kPa or below as not having significant fibrosis, regardless of the other quality indicators. This approach also has the advantage of reducing the proportion of patients classified as having unreliable examinations.
In a study of 754 patients with chronic liver disease and liver histology (349 had NAFLD), our group showed that an absolute CAP IQR of >40 dB/m with M probe measurement was associated with less reliable diagnosis of hepatic steatosis [82]. The finding was confirmed by another study using MRI-PDFF as the reference standard [34], but not in another biopsy-based multicenter United Kingdom study [31]. Nevertheless, the latter study only included patients with suspected NAFLD, with only 47 having grade 0 steatosis. That cohort is thus not well suited to determine the reliability criteria for the diagnosis of fatty liver.
CONFOUNDING FACTORS
Well-studied confounding factors for LSM leading to false-positive diagnosis of advanced fibrosis include hepatic congestion [83,84], biliary obstruction [85], amyloidosis [86], and benign and malignant liver lesions [87,88]. Probably because of increased portal blood flow, liver stiffness increases after meals by 1–5 kPa [89]. The liver stiffness typically peaks at 20 to 40 minutes but may still be increased by 180 minutes.
Acute viral hepatitis and acute exacerbation of chronic viral hepatitis also increase liver stiffness dramatically [90,91]. In fact, patients with chronic hepatitis B and serum alanine aminotransferase (ALT) elevation to one to five times the upper limit of normal also have higher liver stiffness than those with normal ALT [45]. However, ALT elevation does not appear to affect LSM in NAFLD patients [47]. Two reasons may explain this difference. First, NASH is an insidious disease not usually characterized by episodes of acute exacerbations. In general, the degree of hepatic necroinflammation is less prominent in NASH than viral hepatitis or autoimmune hepatitis. Second, the ALT level correlates poorly with histological necroinflammation in NAFLD patients [92].
One controversial point is whether severe hepatic steatosis affects liver stiffness. An Italian study showed that severe steatosis increased the false-positive diagnosis of advanced fibrosis by LSM using the M probe in NAFLD patients [93]. The same applies to patients with high CAP values [42]. However, it is unclear if the effect is directly due to hepatic steatosis. Other studies have also shown that extreme body mass index is associated with higher liver stiffness [94]. Recently, our group performed both M and XL probe measurements on 496 patients with biopsy-proven NAFLD and showed that LSM by the XL probe was not affected by severe steatosis [78].
Because the above factors represent physical properties of the liver parenchyma, they are expected to affect other kinds of LSMs similarly such as by point-shear wave elastography, 2D-shear wave elastography and magnetic resonance elastography. In contrast, confounding factors for CAP have not been as well studied.
GUIDELINE RECOMMENDATIONS
The recommendations of this article are largely in keeping with regional guidelines. The American Association for the Study of Liver Diseases guidelines state that transient elastography and MRE are clinically useful tools for identifying advanced fibrosis in patients with NAFLD, whereas simple fibrosis scores such as the NAFLD fibrosis score and Fibrosis-4 index are clinically useful tools for identifying NAFLD patients with higher likelihood of having bridging fibrosis or cirrhosis [95]. The joint European Association guidelines on NAFLD recommend biomarkers and scores of fibrosis, as well as transient elastography, as acceptable non-invasive procedures for the identification of cases at low risk of advanced fibrosis or cirrhosis [96]. They also suggest the use of a combination of biomarkers, scores and transient elastography to monitor fibrosis progression but highlight that this strategy requires validation. Likewise, the joint European and Latin American guidelines on fibrosis assessment endorse screening of liver fibrosis in high risk populations such as patients with metabolic syndrome or type 2 diabetes [97]. Non-invasive assessment by serum biomarkers or transient elastography can be used as first line procedure for the identification of patients at low risk of severe fibrosis. They also suggest follow-up assessment by serum biomarkers or transient elastography at a 3-year interval. The Asia-Pacific Working Party of NAFLD guidelines also state that non-invasive serum and physical tests have acceptable accuracies when used to measure the fibrotic burden of NAFLD patients [5]. Notably, none of the guidelines specify cutoffs for CAP and LSM.
As discussed above, the Baveno VI consensus statements recommend the use of transient elastography and platelet count to select patients for endoscopic screening for varices [57]. They also suggest the use of simple cutoffs of define low and high probability of compensated advanced chronic liver disease.
CONCLUSIONS
The development of transient elastography has allowed simultaneous and reasonably accurate assessment of hepatic steatosis and fibrosis. The technique is thus well suited as a point-of-care diagnostic and assessment tool for NAFLD patients. CAP and LSM has been validated across different regions and patient populations with consistent results. LSM not only reflects the degree of liver fibrosis but also predicts portal hypertension, varices requiring treatment, cirrhotic complications and hepatocellular carcinoma. While obesity used to be a common reason for measurement failure, it is possible to obtain valid measurements in the majority of NAFLD patients when the XL probe is used in obese patients. Importantly, with the automatic probe selection tool, operators can apply the same liver stiffness cutoffs when the M and XL probes are used for the right patients. Nonetheless, whether the same applies to CAP interpretation deserves further studies. Well-validated reliability criteria for LSM include the requirement of 10 valid measurements and an IQR/M of less than 0.3. Although two studies suggest the IQR also reflects the reliability of CAP, data are conflicting and need further clarification.
As pharmacological treatment for NASH will likely become available in the near future, it is timely to consider the position of non-invasive tests in different settings. Several prospective studies have illustrated the application of simple fibrosis scores, fibrosis biomarkers and transient elastography to detect significant liver diseases at primary care setting and selective populations [39,98]. Notably, it may not be feasible to perform transient elastography for all patients with type 2 diabetes and obesity. A stepwise approach using simple fibrosis scores followed by fibrosis biomarkers or LSM will probably be the way to go but needs to be adjusted for the local setting.
Notes
Authors’ contributions
Xinrong Zhang, Grace Wong and Vincent Wong contributed to the literature review and manuscript preparation.
Conflicts of Interest
Grace Wong has served as an advisory committee member for Gilead Sciences and Janssen, and a speaker for Abbott, AbbVie, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen and Roche. Vincent Wong has served as an advisory committee member or consultant for 3V-BIO, AbbVie, Allergan, Boehringer Ingelheim, Center for Outcomes Research in Liver Diseases, Echosens, Gilead Sciences, Intercept, Janssen, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, TARGET-NASH and Terns, and a speaker for Bristol-Myers Squibb, Echosens, Gilead Sciences, and Merck. Both Grace Wong and Vincent Wong have received unrestricted grants from Gilead Sciences.
Abbreviations
ALT
alanine aminotransferase
AUROC
area under the receiver-operating characteristics curve
BMI
body mass index
cACLD
compensated advanced chronic liver disease
CAP
controlled attenuation parameter
CI
confidence interval
EGD
esophagogastroduodenoscopy
EV
esophageal varices
ICC
intraclass correlation coefficient
IQR-M
interquartile range-to-median ratio
LSM
liver stiffness measurement
MRE
magnetic resonance elastography
MRI-PDFF
magnetic resonance imaging proton density fat fraction
NAFL
nonalcoholic fatty liver
NAFLD
nonalcoholic fatty liver disease
NASH
nonalcoholic steatohepatitis