Endoscopic treatment or balloon-occluded retrograde transvenous obliteration is safe for patients with esophageal/gastric varices in Child-Pugh class C end-stage liver cirrhosis
Article information
Abstract
Background/Aims
There is a controversy about the availability of invasive treatment for esophageal/gastric varices in patients with Child-Pugh class C (CP-C) end-stage liver cirrhosis (LC). We have evaluated the validity of invasive treatment with CP-C end-stage LC patients.
Methods
The study enrolled 51 patients with CP-C end-stage LC who had undergone invasive treatment. The treatment modalities included endoscopic variceal ligation in 22 patients, endoscopic injection sclerotherapy in 17 patients, and balloon-occluded retrograde transvenous obliteration (BRTO) in 12 patients. We have investigated the overall survival (OS) rates and risk factors that contributed to death within one year after treatment.
Results
The OS rate in all patients at one, three, and five years was 72.6%, 30.2%, and 15.1%, respectively. The OS rate in patients who received endoscopic treatment and the BRTO group at one, three, and five years was 67.6%, 28.2% and 14.1% and 90.0%, 36.0% and 18.0%, respectively. The average of Child-Pugh scores (CPS) from before treatment to one month after variceal treatment significantly improved from 10.53 to 10.02 (P=0.003). Three significant factors that contributed to death within one year after treatment included the presence of bleeding varices, high CPS (≥11), and high serum total bilirubin levels (≥4.0 mg/dL).
Conclusions
The study demonstrated that patients with a CPS of up to 10 and less than 4.0 mg/dL of serum total bilirubin levels may not have a negative impact on prognosis after invasive treatment for esophageal/gastric varices despite their CP-C end-stage LC.
Introduction
Portal hypertension in advanced liver cirrhosis (LC) may be caused by various factors including obstruction of the terminal hepatic veins by regenerative nodules, fibrous proliferation of the perisinusoidal space, and sinusoidal narrowing due to hepatocyte degeneration, which increase intrahepatic venous pressure and inflow into the portal vein [1]. Because portal vessels lack venous valves to prevent backflow of blood, a part of portal blood flow that is normally antegrade hepatopetal becomes retrograde hepatofugal, leading to the formation of collateral pathways [2-5]. The growth of collateral pathways and local hyperdynamic state of the upper abdomen in portal hypertension can result in the development of esophageal/gastric varices [6]. Rupture of esophageal/gastric varices may lead to life-threatening and massive bleeding. Therefore, patients with varices at risk of rupture should be treated prophylactically even in cases without bleeding.
It is generally recommended that invasive treatment of esophageal/gastric varices should devise treatment policies very carefully in patients with Child-Pugh class C (CP-C) end-stage LC. This population has a high risk of hepatic failure because of decreased intrahepatic blood flow or portal thrombosis after the procedure [7,8]. However, although invasive treatment is often required in cases of hemorrhagic shock caused by ruptured varices, there is a lack of consensus on its effects.
In the present study we aimed to evaluate the safety and prophylactic effect of variceal treatment with endoscopic procedures and balloon-occluded retrograde transvenous obliteration (BRTO) in advanced LC.
Materials and Methods
The study included 51 patients with CP-C end-stage LC who underwent endoscopic procedures or BRTO for esophageal/gastric varices between April 1995 and December 2017. The characteristics of the study population are shown in Table 1. There were 40 men and 11 women, with a mean age of 60.4±10.7 years. The etiology of LC was hepatitis C virus infection in 19 patients, hepatitis B virus infection in 6 patients, alcohol injury in 18 patients, and others in 8 patients. The Child-Pugh scores (CPS) in each etiology were 10 in 35 patients, 11 in 8 patients, 12 in 3 patients, 13 in 3 patients, and 14 in 2 patients, respectively. The purpose of treatment was bleeding in 31 patients, prophylaxis in 20 patients. The treatment procedure was endoscopic variceal ligation (EVL) in 22 patients, endoscopic injection sclerotherapy (EIS) in 17 patients (5% ethanolamine oleato injection in 10 patients and α-cyanoacrylate monomer injection in 7 patients) and BRTO in 12 patients. The survival time was less than 1 year in 21 patients, more than 1 year in 22 patients, and unknown in 8 patients.
Clinical variables were analyzed in three categories: (1) the effect of all procedures on patient outcomes as assessed by the overall survival (OS) rates in all patients; (2) respective outcomes in patients according to the type of procedures (endoscopic treatments and BRTO) as assessed by the OS rates in the endoscopic treatment group (n=39) and the BRTO group (n=12) in all patients, and by changes in CPS; and, (3) factors contributing to death within 1 year of treatment.
Our research protocol was approved by the Ethics Committee of Fukuoka University Hospital (Approval No.16-1-16).
Statistical analyses were performed using JMP version 10.0 (SAS Institute, Cary, NC, USA). The methods of changes in each parameter and comparison between groups included Wilcoxon signedrank test, the t -test and chi-square test. The cumulative survival curves were calculated according to the Kaplan-Meier method, a log-rank test was used for the comparison between each curve. P -values less than 0.05 were considered significant.
Results
The OS rates in all 51 patients at 1, 2, 3 and 5 years were 72.6%, 50.1%, 30.2% and 15.1%, respectively (Fig. 1). The characteristics of the endoscopic treatment group and the BRTO group were shown in Table 2. The OS rates at 1, 2, 3 and 5 years were 67.6%, 43.9%, 28.2% and 14.1% in the endoscopic treatment group, respectively (Fig. 2A, solid line). There was no significant difference in OS between 22 patients treated with EVL and 17 patients treated with EIS (P =0.262, figure not shown). The OS rate at 1, 2, 3 and 5 years were 90.0%, 72.0%, 36.0% and 18.0% in the BRTO group, respectively (Fig. 2A, dotted line). There were no significant differences in the survival rates between the groups. The OS rates in patients with bleeding variceal treatment at 1, 2 and 3 years were 41.2%, 33.6% (Fig. 2B, solid line), and 13.2% in the endoscopic treatment group and 26.7%, 26.7% and 26.7% in the BRTO group, respectively (Fig. 2B, dotted line).

The overall survival (OS) rates in all patients (n=51). The OS rates in all 51 patients at 1, 2, 3 and 5 years were 72.6%, 50.1%, 30.2% and 15.1%, respectively.
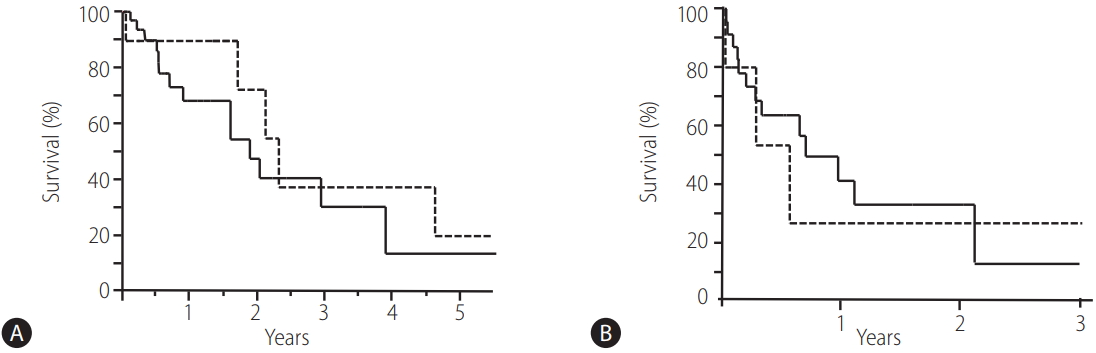
In a comparison of the overall survival (OS) rates in the endoscopic treatment group and in the BRTO group. (A) The OS rates in the endoscopic treatment group (n=39) and in the BRTO group (n=12). The OS rates at 1, 2, 3 and 5 years were 67.6%, 43.9%, 28.2% and 14.1% in the endoscopic treatment group (solid line) and 90.0%, 72.0%, 36.0% and 18.0% in the BRTO group (dotted line), respectively. There were no significant differences in the survival rates between the groups. (B) The OS rates in patients with bleeding varices in the endoscopic treatment group (n=26) and the BRTO group (n=5). The OS rates in patients with bleeding variceal treatment at 1, 2 and 3 years were 41.2%, 33.6% and 13.2% in the endoscopic treatment group (solid line) and 26.7%, 26.7% and 26.7% in the BRTO group (dotted line), respectively. There were no significant differences in the survival rates between the groups. BRTO, balloon-occluded retrograde transvenous obliteration.
45 of 51 patients with variceal treatment were able to follow-up one month after treatment. Changes in CPS and their associated parameter from before treatment to 1 month after treatment were shown in Figure 3. The average of serum prothrombin activity and serum albumin levels was significantly improved (P =0.046 and 0.019), and the average of CPS was significantly improved from 10.53 to 10.02 (P =0.003) from before treatment to 1 month after treatment. The comparison of the mean change ratio in CPS and their associated parameter from before treatment to 1 month after treatment between the endoscopic treatment group (n=37) and the BRTO group (n=8) were shown in Figure 4. The average of serum prothrombin activity was significantly improved in the BRTO group (P =0.019). Meanwhile, ascites was significantly to become worse in the BRTO group (P =0.014). Comprehensively, there was no significant difference of the mean change ratio in CPS between the groups.
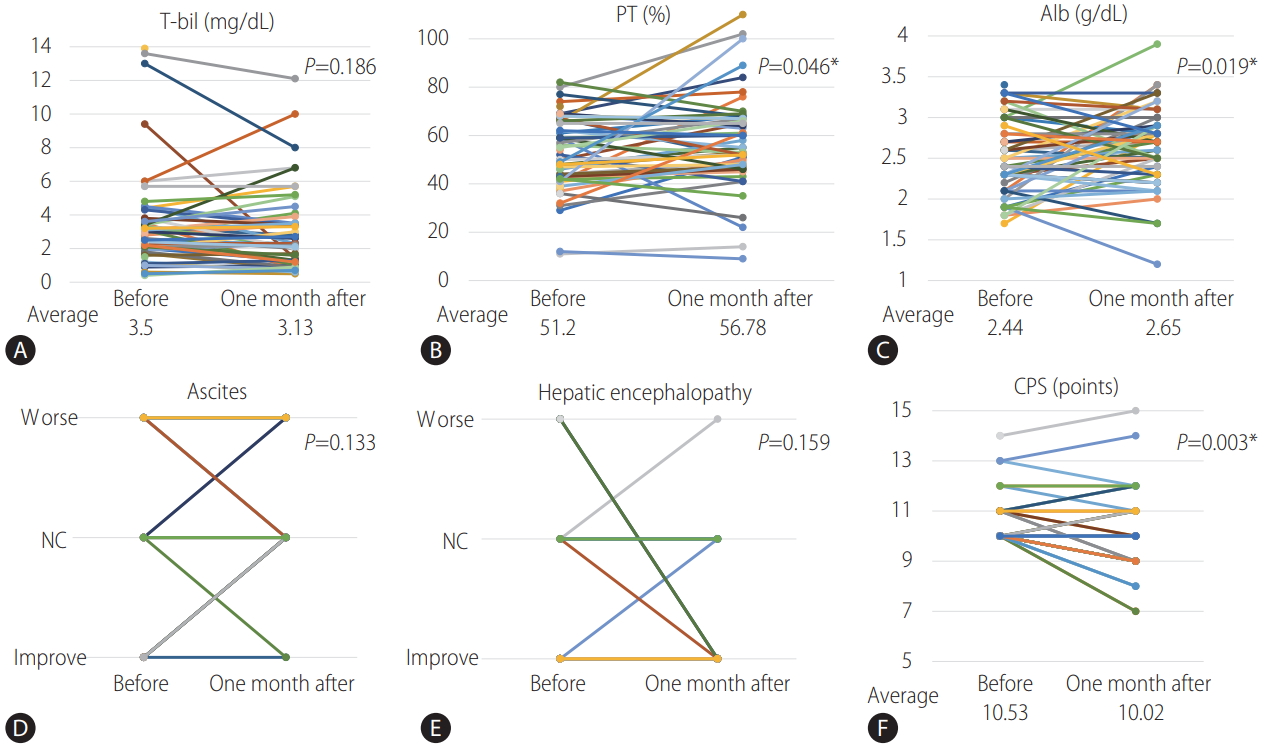
Changes in CPS and their associated parameters from before treatment to one month after treatment for varices (n=45). (A) T-bil. (B) PT activity. (C) Alb. (D) Ascites. (E) Hepatic encephalopathy. (F) CPS. T-bil, total bilirubin; PT, prothrombin activity; Alb, albumin; NC, no change; CPS, ChildPugh Score. * P<0.05 was considered statistically significant.
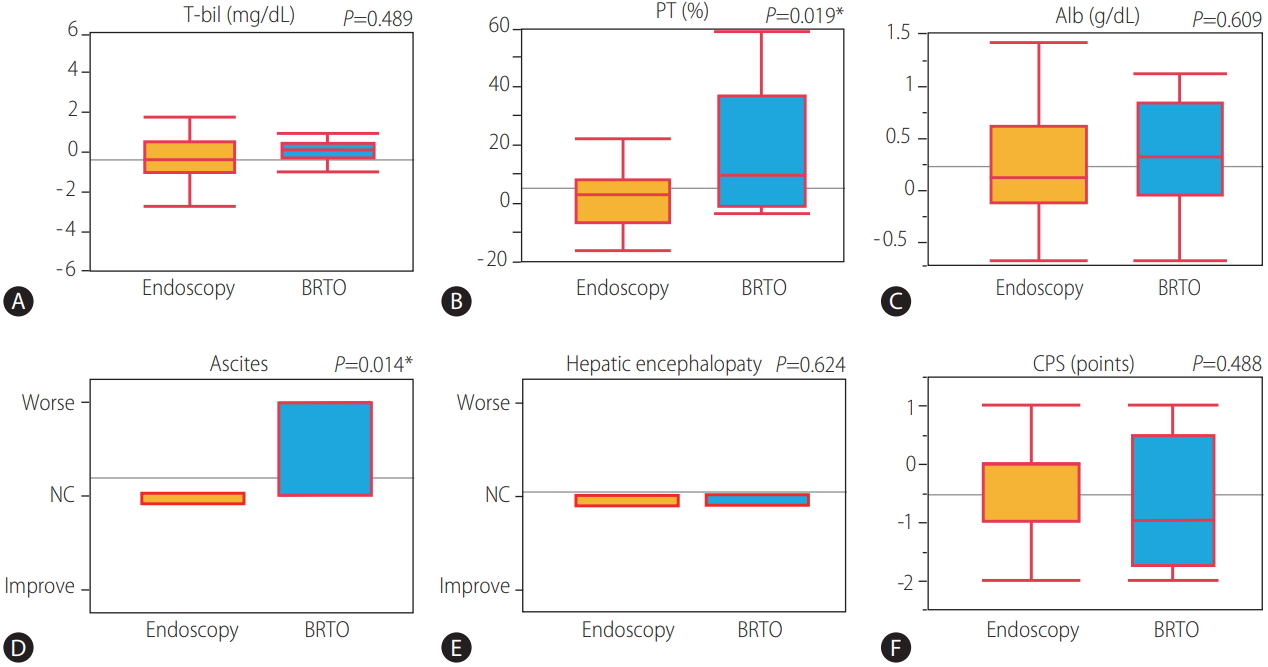
In a comparison of the mean change ratio in CPS and the associated parameters from before treatment to one month after variceal treatment between the endoscopic treatment group (n=37) and the BRTO group (n=8). (A) T-bil. (B) PT activity. (C) Alb. (D) Ascites. (E) Hepatic encephalopathy. (F) CPS. T-bil, total bilirubin; PT, prothrombin activity; Alb, albumin; BRTO, balloon-occluded retrograde transvenous obliteration; NC, no change; CPS, Child-Pugh Score. * P<0.05 was considered statistically significant.
As shown in Table 3, univariate analysis of factors contributing to death within 1 year of treatment identified three significant variables: the presence of bleeding varices, high CPS (≥11) and high serum total bilirubin levels (≥4.0 mg/dL). The presence of hepatocellular carcinoma (HCC) was not significantly associated with death within 1 year of treatment.
Discussion
A previous important systematic review reported that any studies regarding predictors of death should include the CPS and age in patients with decompensated cirrhosis [9]. This large systematic review demonstrated that the OS rates at 1, 2, 3 and 5 years were approximately 60%, 45%, 35% and 25% in a large cohort of decompensated LC, respectively. Moreover, the OS rates were 45% at 1 year and 38% at 2 years in patients with CP-C end-stage LC [9]. According to a recent report in Japan, the cumulative 2-year survival rates of CP-C patients in the liver transplantation registry were 38.1% [10]. Compared with these reports, the OS rates after treatment in all patients, and in each treatment group were higher than those in the present study. A reason for the better survival outcome may be associated with the relatively large proportion of patients with a CPS of 10. In addition, CPS was significantly decreased after variceal treatment in the present study. It can be explained by that liver function could be improved by multidisciplinary treatment for cirrhosis after invasive treatment, even in patients with CP-C end-stage LC.
BRTO was first described by Kanagawa et al. in 1996 as a treatment for isolated gastric varices [11]. Although there are concerns about the adverse effects of BRTO, including renal dysfunction by hemolysis, worsening of esophageal varices, and increased ascites, it has been reported that BRTO contributed to improve survival [12-18]. In the comparison of the mean change ratio in CPS and their each parameter from before treatment to 1 month after treatment between the endoscopic treatment group and the BRTO group, the average of prothrombin activity was significantly improved in the BRTO group, meanwhile ascites was significantly worsen in the BRTO group. Comprehensively, there was no significant difference of the mean change ratio in CPS between the groups. Previous studies have shown that occlusion of a large portosystemic shunt by BRTO increases the effective portal blood flow into the liver and improves hepatic functional reserve [19-23] which is likely to contribute to improve survival rates in the present study. In contrast, BRTO has been linked to the risk of portal vein thrombosis or impaired intrahepatic vascular perfusion by outflow of ethanolamine oleate to portal veins or the lung, subsequent hepatic failure, decreased respiratory function [24], and balloon rupture [25]. Therefore, for BRTO to be implemented safely and effectively, it is necessary to learn the appropriate technical skills. Recently, form-BRTO [26,27] or combination therapy with BRTO and partial splenic embolization [28] was found to safely improve hepatic function by reducing the use of 5% ethanolamine oleate.
In the present study, there was no significant difference in the survival rates between patients treated with EVL versus EIS. The mean dose of 5% ethanolamine oleate used for EIS was 9.1±5.80 mL, which was set at approximately 0.2 mL/kg, a relatively low dose considering hepatic functional reserve. This dose setting could be responsible for the lack of difference in the survival rates between EVL and EIS despite the less invasive nature of EVL.
In the present study, the presence of HCC was not a significant factor contributing to death within 1 year of treatment. Because none of the patients with HCC had severe portal invasion of portal vein invasion of the second branches or higher, it is possible that hepatic failure, rather than cancer progression, was the main cause of death in most cases.
Another finding was that three variables such as the presence of bleeding varices, higher CPS (11 or higher) and higher serum total bilirubin levels (4.0 mg/dL or higher) were significantly related to an increased risk of death within 1 year of treatment. However, in patients with bleeding, it is difficult to determine whether the cause of early death is the invasiveness of treatment itself or the reduced hepatic functional reserve due to bleeding and ischemia. The present results suggested that CP-C end-stage LC patients with esophageal/gastric varices and/or hepatic encephalopathy which required treatment, together with advanced jaundice (serum total bilirubin levels of 4.0 mg/dL or higher) and/or high CPS (11 or higher), have a poor prognosis after treatment. Clinicians should be aware of these factors to enable them to inform patients and to perform treatment in an optimal manner. Complications of EIS or BRTO can easily lead to hepatic failure, therefore continuous efforts are necessary to perform procedures skillfully and to establish a safe and effective standard treatment.
A limitation of the present study design is not being a comparative/randomized trial. Various patients with heterogenous background (etiology of cirrhosis, purpose of treatment, history of variceal bleeding) were enrolled. Therefore it is difficult for effective comparison of clinical usefulness between endoscopic procedure and BRTO. Furthermore, in this study, only univariate analysis was performed, not multivariate analysis because of the small sample size. It is necessary to analyze data from a larger population in future studies.
In conclusion, there is a conflicting research about invasive treatments such as endoscopic procedures and BRTO for patients with CP-C end-stage LC. The findings of this study suggested that, among patients with CP-C end-stage LC, those with lower CPS (10 or lower) and lower serum total bilirubin levels (4.0 mg/dL or lower) did not have a worse prognosis after invasive treatment.
Acknowledgements
The authors gratefully acknowledge Prof. Kengo Yoshimitsu, Chairman in the Department of Radiology, Fukuoka University, and Associate Prof. Hideyuki Higashihara for performing BRTO procedures in the Department of Radiology, Fukuoka University Chikushi Hospital.
Notes
Authors’ contribution
K.Y. designed and carried out the study; All authors analyzed the data; K.Y. wrote the paper.
Notes
Conflicts of Interest: The authors declare that there is no conflict of interests regarding the publication of this paper.
Abbreviations
BRTO
balloon-occluded retrograde transvenous obliteration
CP-C
Child-Pugh class C
CPS
Child-Pugh scores
EIS
endoscopic injection sclerotherapy
EVL
endoscopic variceal ligation
HCC
hepatocellular carcinoma
LC
liver cirrhosis
OS
overall survival
References
Article information Continued
Notes
Study Highlights
The study evaluated the validity of endoscopic treatment and balloon-occluded retrograde transvenous obliteration for esophageal/gastric varices in patients with Child-Pugh class C end-stage liver cirrhosis. The study demonstrated that patients with a Child-Pugh scores of up to 10 and less than 4.0 mg/dL of serum total bilirubin levels may not have experienced a negative impact on prognosis after invasive treatment.