Comparison of surgical resection versus transarterial chemoembolization with additional radiation therapy in patients with hepatocellular carcinoma with portal vein invasion
Article information
Abstract
Background/Aims
Portal vein invasion (PVI) is a poor prognostic factor in patients with hepatocellular carcinoma (HCC). We intended to compare the effects of surgical resection and transarterial chemoembolization (TACE) with additional radiation therapy (RT) in HCC patients with PVI.
Methods
The subjects comprised 43 patients who underwent surgical resection for HCC with PVI without previous treatment and another 43 patients who received TACE followed by RT (TACE+RT) as initial treatment who were matched for Child-Pugh class, tumor size, and extent of PVI. Disease progression and death after the treatment were examined, and progression-free survival (PFS) and overall survival (OS) were compared between groups. Predisposing factors affecting OS were analyzed using univariate and multivariate analyses in HCC patients with PVI.
Results
The subjects (Age [51, 24-74; median, range], Sex [81/13; male/female], Etiology [78/1/15; hepatitis B virus {HBV}/ hepatitis C virus {HCV}/non-HBV and non-HCV]) were followed for a median of 17 (2-68) months. There were no differences in clinical or tumor characteristics between the resection and TACE+RT groups. The cumulative PFS was not significantly different between groups. The median PFS was 5.6 and 4.0 months in the resection and TACE+RT groups, respectively. However, the cumulative OS was significantly longer in patients treated with resection than in those treated with TACE+RT (P=0.04). The median OS was 26.9 and 14.2 months in the resection and TACE+RT groups, respectively. Univariate and multivariate analyses revealed that surgical resection was an independent predictive factor for better survival outcome.
Conclusions
Surgical resection might be an effective treatment in HCC patients with PVI.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third major cause of malignant tumor-related death worldwide [1]. In a considerable number of patients, HCC is initially diagnosed in the advanced stage, thus eliminating the opportunity for curative treatment such as resection, radiofrequency ablation or transplantation. Portal vein invasion (PVI) is one of the most important prognostic factors in patients with HCC and is found in approximately 12-40% of these patients [2-5].
The current treatment guideline from the Barcelona Clinic Liver Cancer system recommends palliative systemic therapy such as sorafenib for HCC with PVI [6,7]. However, in Asian-Pacific countries, transarterial chemoembolization (TACE) has been widely used as the first-choice treatment in patients with advanced-stage HCC since before sorafenib became available.
Moreover, owing to the challenge for more aggressive treatment and advances in medical techniques, TACE or surgical resection are no longer contraindications for HCC with PVI [5,8-10]. Particularly, TACE with additional radiation therapy (RT) has been suggested to be safer and more beneficial to survival than TACE alone for HCC with PVI [8,11,12]. Surgical resection can also be considered an effective treatment strategy in selected patients [5,13-15].
The optimal treatment in HCC patients with PVI is still controversial. Thus, in this study, we aimed to compare and analyze the treatment efficacy between TACE with additional RT (TACE+RT) and resection in HCC patients with PVI.
PATIENTS AND METHODS
Patients and methods
The subjects comprised a total of 86 HCC patients with PVI (43 in resection group vs. 43 in TACE+RT group). In order to recruit resection group, 54 patients who underwent surgical resection for HCC with PVI between 2005 and 2008 at Asan Medical Center were screened. Of them, 43 patients who received surgical resection as a primary treatment were subjected. Three hundred eighty one patients who received TACE+RT for HCC with PVI during the same period were also screened. And then, 43 patients initially treated with TACE+RT were selected with matching Child-Pugh class (A vs. B), tumor size, and extent of PVI (main or bilateral vs. unilateral). In all patients, HCC was radiologically or histologically diagnosed on the basis of the practice guidelines of the American Association for the Study of Liver Diseases [6]. In addition, all patients had no extrahepatic metastasis at baseline. The patients in the resection group additionally fulfilled the following criteria: 1) no involvement of the resection margin and 2) complete tumor removal including the portal vein tumor thrombus. In the TACE+RT group, TACE was performed first and then RT was done subsequently within 1 month after initial TACE. The detailed procedures of TACE and RT were described in our previous report [8].
The clinical information of patients including demographics, etiologies of liver disease, and laboratory data were obtained at the time of diagnosis. Tumor characteristics such as the extent of PVI, radiologic morphology, and number of tumors were also evaluated initially. The patients were followed at intervals of 1-3 months, and recurrence, progression, and patients’ survival were evaluated through history taking, physical examination, laboratory findings, and imaging modalities such as a dynamic computed tomography (CT) scan or magnetic resonance images (MRI) at each visit. For patients in the TACE+RT group, repeated TACEs were performed based on the findings of CT or MRI, patients’ performance status, and hepatic reserves.
Definitions
Progressive disease was included under the following conditions: recurrence defined as new detection of HCC in the liver or extrahepatic lesions in the resection group, or an increase of at least 20% in the diameter of viable tumor or newly developed HCC according to the modified Response Evaluation Criteria in Solid Tumors criteria in the TACE+RT group. Progression-free survival (PFS) and overall survival (OS) were measured from the time of diagnosis of HCC with PVI to the date of HCC progression and death or last visit, respectively. The study endpoints were progression of HCC and the OS.
Statistical analyses
The variables were compared using the chi-square test, Fisher’s exact test, or Student’s t test. The OS and PFS curves were estimated with the Kaplan-Meier method and compared statistically using the log-rank test. Statistical analyses were conducted using SPSS software package (version 22; IBM Corp., Armonk, NY, USA).
RESULTS
Baseline characteristics
The baseline characteristics of the resection and TACE+RT groups are shown in Table 1. The demographic, clinical, and laboratory characteristics, including age, sex, etiology, Child-Pugh classification, and serum alpha-fetoprotein (AFP), were similar between the two groups. The tumor characteristics such as size, number, morphology, and stage were also not different between the two groups. The median follow-up duration was 22 months (range, 3-68 months) in the resection group and 14 months (range, 2-46 months) in the TACE+RT group. The follow-up duration of the resection group was significantly longer than that of TACE+RT group (P =0.01).
Responses to each treatment
There was no treatment-related mortality in both the resection and the TACE+RT groups. However, in the resection group, one patient suffered from pleural effusion within one month after surgery. In the TACE+RT group, chronic hepatitis B flare-up and the complications of cirrhosis such as jaundice and spontaneous bacterial peritonitis were developed in 3 and 2 patients, respectively. All of the patients who had treatment-related complications were improved after proper management.
During the follow-up, the proportion of patients who achieved progressive disease was 86% (n=37) for the resection group and 81% (n=35) for the TACE+RT group. Intrahepatic progression was identified in 62 patients (72%), and extrahepatic metastasis was found in 52 patients (61%). There were no significant differences in intrahepatic and extrahepatic progression between the two groups. The common metastatic sites were the lungs (n=40), lymph nodes (n=9), and bones (n=5). Of the entire patients, 27 (63%) in the resection group and 32 (74%) in the TACE+RT group have died during the follow-up period.
Among the patients with progressive disease, 34 and 35 patients were received the salvage treatment in the resection and the TACE+RT groups, respectively. In the resection group, most common method of salvage treatment was TACE (n=26), followed by RT (n=3), systemic chemotherapy (n=3), RFA (n=1), and surgery (n=1). Similarly, in the TACE+RT group, TACE (n=28) was most common, followed by surgery (n=4) and RT (n=3).
Progression-free and overall survival
The PFS rates did not show significant differences between the resection and the TACE+RT groups. The 1- and 3-year PFS rates were 23% and 16%, respectively, in the resection group. Similarly, patients in the TACE+RT group showed the 1-year PFS rate of 26% and 3-year PFS rate of 15% (Fig. 1). The median PFS was 5.6 months in patients treated with liver resection and 4.0 months in those who received TACE+RT.
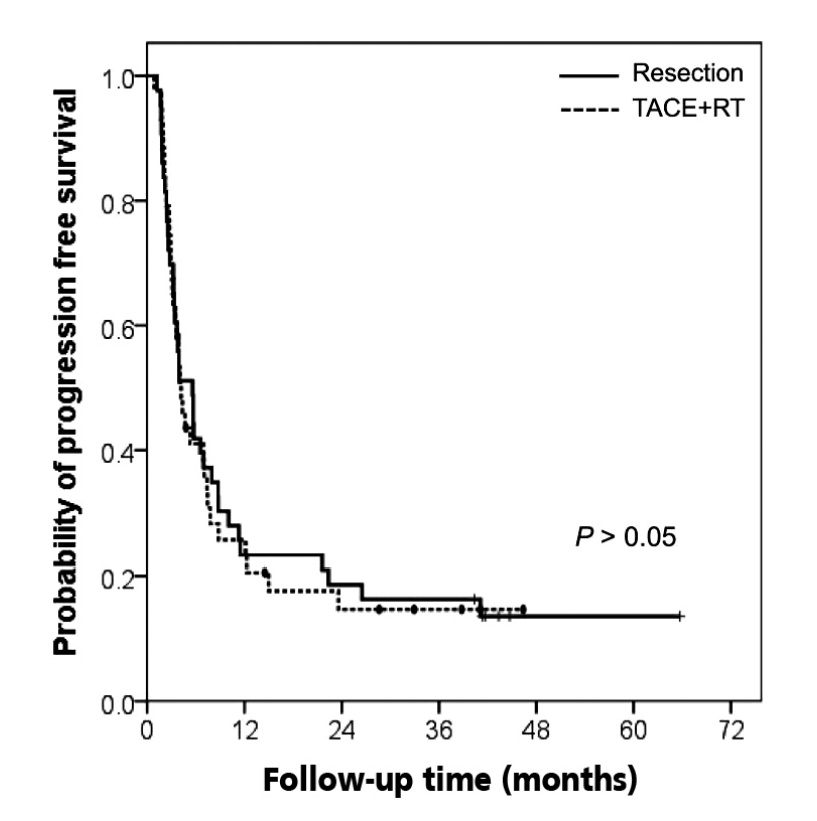
Kaplan-Meier estimates for progression-free survival in relation to treatment group. TACE, transarterial chemoembolization; RT, radiation therapy.
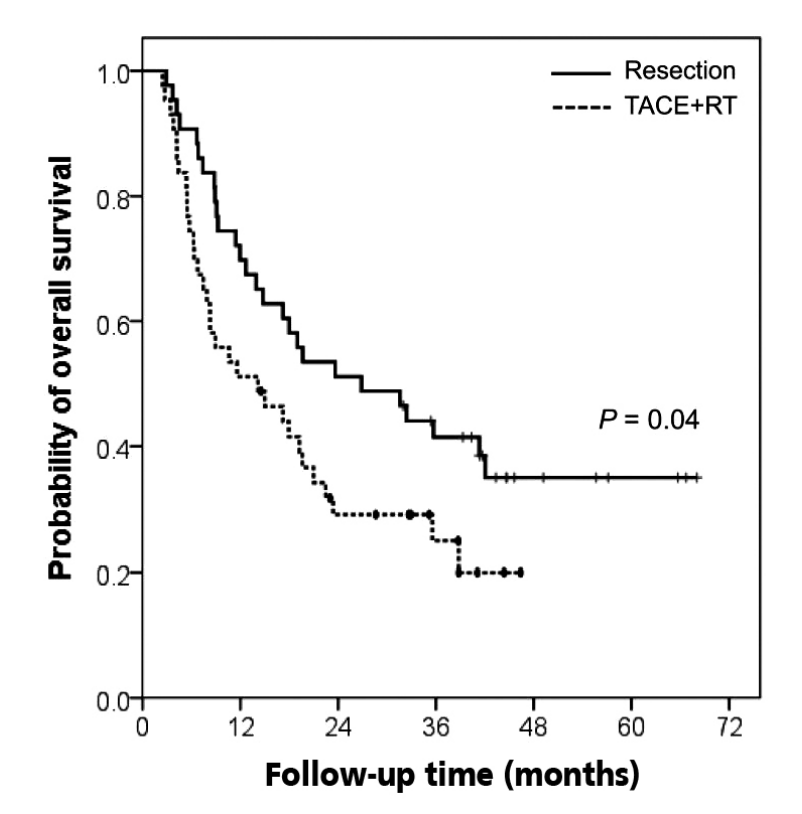
Out of the patients treated by surgery, 27 (63%) died and 32 patients (74%) died in the TACE+RT group during the follow-up. The OS was significantly longer in the resection group than in the TACE+RT group (P =0.04) (Fig. 2). The 1- and 3-year OS rates were 70% and 42% in the resection group and 51% and 25% in the TACE+RT group, respectively. The median OS was 26.9 months in the resection group and 14.2 months in the TACE+RT group.
Predictors of PFS and OS
Factors affecting PFS were evaluated using univariate analyses in HCC patients with PVI. However, the initial treatment modalities did not affect PFS. Moreover other characteristics also did not influence PFS (Table 2).
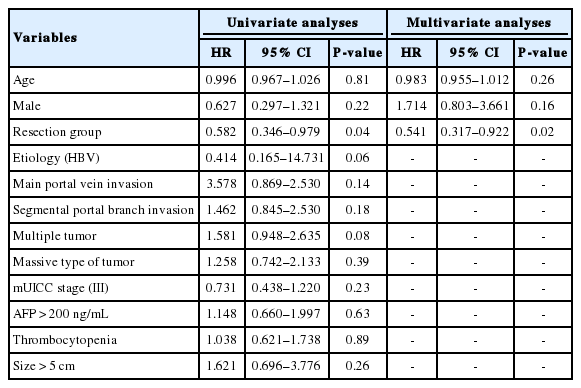
We also evaluated the predictors affecting OS rates in HCC patients with PVI. On univariate analyses, surgical resection was a significant predictive factor associated with longer survival rates [hazard ratio (HR) 0.58, 95% confidence interval (CI) 0.346-0.979; P =0.04; Table 3]. However, the extent of PVI was not an important factor affecting patient survival in this study. Furthermore, we could not find the association between OS rates and other clinical and tumor characteristics such as sex, age, etiology of underlying liver disease, number of tumors, and tumor morphology (Table 3). Age- and sex-adjusted analysis also revealed that surgical resection was an independent factor predicting longer survival rates in HCC patients with PVI (HR 0.54, 95% CI 0.317-0.922; P =0.02; Table 3).
DISCUSSION
In this study, we compared the treatment efficacy between surgical resection and TACE followed by RT in patients with HCC and PVI. Our study demonstrated that patients who received surgical resection achieved higher survival rates than those treated with TACE+RT.
PVI is one of the most common complications of HCC. The presence of PVI in HCC is classified as Barcelona Clinic Liver Cancer stage C, and sorafenib has been recently recommended as a treatment for patients with this condition [16]. Sorafenib provided significant improvement in OS and increased the median OS from 4.2 to 6.5 months compared with placebo in an Asia-Pacific trial [17]. In the subset analysis of this trial, the median OS (5.6 vs. 4.1 months) was slightly longer in patients with macrovascular invasion who received sorafenib than in patients in the placebo group [18].
However, in Asian-Pacific countries where the HCC prevalence is high, variable treatment options were attempted before sorafenib was approved. Surgical resection for HCC with PVI was initially reported in 1990 [19]. Thereafter, many studies have demonstrated that hepatic resection has potential benefits on the OS or recurrencefree survival of patients with HCC and PVI [13,14,20-22].
Traditionally, TACE has been widely performed for HCC treatment, regardless if the tumor is in the very early or advanced stage. However, the therapeutic effects of TACE have not been satisfactory for HCC with PVI although this procedure has been generally and safely applied. For this reason, the addition of RT to TACE has been attempted for these patients. RT for HCC was previously used restrictively owing to the limitation of poor radiation tolerance and, consequently, the small effects of RT [8,23]. However, the rapid advancement of techniques has made RT feasible and well tolerated in patients with HCC, thereby considerably improving the disease control rates of RT. In patients with advanced HCC such as vascular invasion, a combination strategy of TACE for intrahepatic HCC and RT for vascular invasion of the tumor has been performed, and effective results were reported compared with TACE alone [8,23]. Particularly, Kim et al. reported the efficacy of TACE+RT in HCC patients with PVI [8]. The study revealed that patients treated with RT+TACE had a 1-year survival rate of 33.3% and 63.6%, respectively, in the groups of main PVI and branch PVI with Child-Pugh A, whereas those treated with TACE alone had 12.5% and 35.6%.
Practically, we have attempted TACE+RT or surgical resection for patients with HCC and PVI when sorafenib was not available in Korea. Nowadays we still frequently considered these treatment options as well as sorafenib as an initial treatment. However, there were no data to compare the effects between surgical resection and TACE followed by RT in HCC patients with PVI. Our present study showed that surgical resection was more effective than TACE+RT in improving patient survival. Our study did not reveal a significant difference of PFS between the resection group and the TACE+RT group. As expected, the tumor recurrence or progression rates were still high in patients with advanced HCC regardless of treatment modalities. In the resection group, however, recurrence especially intrahepatic recurrence might be more controllable than those in TACE+RT group due to the relatively small extent of recurred tumor. Moreover, TACE was continuously performed in considerable number of patients in the TACE+RT group, although they had the progressive disease after TACE and RT. Thus the response of salvage treatment may also be better in resection group than those of TACE+RT group. Actually, some of the patients achieved the complete remission after salvage treatment in the resection group (not shown data). Consequently, we could suggest that this effective control of recurred tumor might result in a survival benefit in patients treated by resection.
Although we found that resection for HCC with PVI was superior over TACE+RT, the choice of surgical indication should be made carefully. According to previous reports, the surgical indications for these patients are still controversial [21,22,24]. Nonetheless, it is clear that individual hepatic reserves, degree of portal hypertension, and technical availability are the factors that need special considerations.
Our study has potential limitations. The data were analyzed retrospectively and the sample size was relatively small. Thus, to minimize the selection bias and overcome these shortcomings, the subjects of each group were enrolled by matching for underlying liver function, tumor size, and PVI extent. Despite our efforts, the possibility of other unconsidered might exist. Thus, we agree that a further large-scale study is needed prospectively. In conclusion, surgical resection might be associated with better survival outcome than TACE and additional RT in HCC patients with PVI.
Notes
Authors’ contribution
Study conception and design: Han Chu Lee
Acquisition of data: Danbi Lee and Jihyun An
Analysis and interpretation of data: Danbi Lee and Han Chu Lee
Drafting of manuscript: Danbi Lee and Han Chu Lee
Critical revision: Han Chu Lee, Ju Hyun Shim, Kang Mo Kim, Young-Suk Lim, Young-Hwa Chung and Yung Sang Lee
Conflict of Interest
The authors have no conflicts to disclose.
Abbreviations
AFP
alpha-fetoprotein
HCC
hepatocellular carcinoma
OS
overall survival
PFS
progression-free survival
PVI
portal vein invasion
RT
radiation therapy
TACE
transarterial chemoembolization