Validation of P2/MS for reflecting hepatic fibrosis in patients with hepatocellular carcinoma
Article information
Abstract
Background/Aims
P2/MS is known as a simple, accurate, and noninvasive marker for determination of the degree of hepatic fibrosis in patients with viral hepatitis. We aimed to validate P2/MS in patients with HCC.
Methods
Consecutive HCC patients who underwent surgical resection between June 2007 and March 2009 at Seoul National University Hospital were enrolled. Fibrosis stage was reviewed and assessed according to METAVIR scoring. P2/MS values [platelet count (109/L)]2/[monocyte fraction (%)×segmented neutrophil fraction (%)] and other noninvasive fibrosis scoring systems were calculated.
Results
A total of 171 patients were included; seven patients with METAVIR F1, 31 with F2, 41 with F3, and 92 with F4. The area under the receiver-operating characteristic curve of P2/MS was 0.804 [95% confidence interval (CI), 0.681~0.927] for detection of significant fibrosis (F2-F4) and 0.769 (95% CI, 0.698~0.839) for detection of histological cirrhosis (F4). At a value < 62, P2/MS detected significant fibrosis with a specificity of 85.7% (95% CI, 42.0~99.2) and a positive likelihood ratio of 4.268 (95% CI, 0.692~26.309); and at a value > 115, P2/MS ruled out significant fibrosis with a sensitivity of 90.2% (95% CI, 84.4~94.1) and a negative likelihood ratio of 0.34 (95% CI, 0.106~0.095). P2/MS had a superior efficacy for detection of hepatic fibrosis in patients with HCC compared to the other noninvasive panels.
Conclusions
P2/MS can accurately detect fibrosis in patients with HCC. Thus, P2/MS might be utilized as a noninvasive index reflecting the degree of hepatic fibrosis in HCC patients.
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is the main cause of hepatocellular carcinoma (HCC).1 Although it has been reported that up to 40% of HBV-related HCC develops in patients who do not have cirrhosis, almost all cases of chronic hepatitis C virus (HCV) related HCC occur in cirrhotic liver.1 In most cases of HCC which originate from cirrhotic liver, hepatic fibrosis might be one of the early changes of hepatocarcinogenesis because cirrhotic changes of the liver could be resulted from accumulation and exacerbation of fibrosis.2 On the other hand, it has been demonstrated that HCC patients with advanced fibrosis had shorter overall survival periods than those without it.3 The fibrosis score is recommended for tumor-node-metastasis staging of HCC.4 In addition, the Barcelona Clinic Liver Cancer (BCLC) classification integrated several prognostic factors including portal hypertension which is related to hepatic fibrosis.5 Therefore, the accurate evaluation of hepatic fibrosis is necessary not only for determining the plan of antiviral therapy but also for the prognostic value in patients with HCC. The gold standard for assessment of hepatic fibrosis has been liver biopsy.6 However, over- or underestimation of fibrosis can be possible due to intra- and interobserver variability and sampling errors.7,8 Several limitations, such as invasiveness, risk of complication, and the availability of expert practitioners, hamper the clinical use of liver biopsy especially when repeated tests are required.9 The current diagnostic strategy for HCC mostly depends on imaging modalities without liver biopsy.10 Assessment modalities of hepatic fibrosis status without biopsy are required to help predict the prognosis of patients with HCC. Consequently, alternative noninvasive methods based on imaging studies11,12 and biochemical tests13-16 have been suggested as markers of hepatic fibrosis. However, these tests are not able to fully replace liver biopsy due to the high cost or low diagnostic accuracy.17
Recently, a simple, accurate, and noninvasive test for hepatic fibrosis known as P2/MS was developed.17 It uses only simple laboratory tests, i.e., complete blood cell counts.17,18 It was found to have high diagnostic accuracy for reflecting hepatic fibrosis and low cost. The favorable qualities of P2/MS were reconfirmed by an external validation.16 Its clinical application for detecting esophageal varices was assessed by a prospective study.19 However, P2/MS was developed and validated in virus-related chronic liver disease (CLD) patients without HCC.17-19 The applicability of P2/MS in patients with HCC has not yet been validated. In this study, we aimed to validate the diagnostic accuracy of P2/MS and compare it to those of other noninvasive fibrosis scoring systems in patients with HCC.
PATIENTS AND METHODS
Patients
This is a retrospective cohort study. We initially enrolled 177 consecutive HCC patients who underwent surgical resection at Seoul National University Hospital, Seoul, Korea, between June 2007 and March 2009. We excluded six patients whose surgical specimens were not available for evaluating the fibrosis grade of non-tumorous liver parenchyma. A total of 171 patients were included in this study. HCC was diagnosed by imaging modalities with/without alpha-fetoprotein levels based on the American Association for the Study of Liver Diseases guidelines.10 Tumor staging was performed in accordance with the American Joint Committee on Cancer (AJCC) staging system 6th edition.4 This study was reviewed and approved by the Institutional Review Board of Seoul National University Hospital.
P2/MS and Other noninvasive fibrosis scoring systems
Laboratory data were acquired from the included patients' medical records. All noninvasive fibrosis scoring systems were calculated using pre-operative laboratory data. The P2/MS values and other noninvasive fibrosis scoring systems were calculated with a previously published formula as following.17,20-23
P2/MS=[platelet count (109/L)]2/[monocyte fraction (%)×segmented neutrophil fraction (%)]
FIB-4=[Age (years)×aspartate transaminase (AST) (U/L)]/[platelet count (109/L)×[alanine transaminase (ALT) (U/L)]1/2]
Goteborg University Cirrhosis Index (GUCI)=(AST/upper limit of normal)×international normalization ratio (INR)×100/[platelet count (109/L)]
AST to platelet ratio index (APRI)=[(AST/upper limit of normal)/platelet count (109/L)]×100
AST to ALT ratio (AAR)=AST/AL
Histological examination of liver tissue
The liver surgical specimens were fixed in formalin and embedded in paraffin. Hematoxylin and eosin (H&E) and Masson's trichrome staining were performed. Histological examinations were carried out by an experienced liver pathologist who was blinded to any clinical information. Fibrosis stage was reviewed and assessed in non-tumorous liver parenchyma according to the METAVIR scoring system.24 Fibrosis was staged on a scale of 0~4 as following: F0=no fibrosis, F1=portal fibrosis without septa, F2=few septa, F3=numerous septa without cirrhosis, and F4=cirrhosis. Significant and severe fibrosis was defined as METAVIR fibrosis scores of F2-F4 and F3-F4, respectively.
Statistical analysis
Spearman's rank correlation coefficient was calculated to measure the correlation between P2/MS scores and the degree of fibrosis. Receiver operating characteristic (ROC) curves were constructed and the area under the ROC curve (AUROC) was calculated to assess the diagnostic accuracy of P2/MS. The AUROC of P2/MS for detecting significant fibrosis, severe fibrosis, and histological cirrhosis were calculated. The sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR) were then calculated using the ROC curves at different cut-off points. All analyses were conducted using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) and STATA version 10.0 (STATA Corp., College Station, TX, USA). P < 0.05 was considered significant.
RESULTS
Patient characteristics
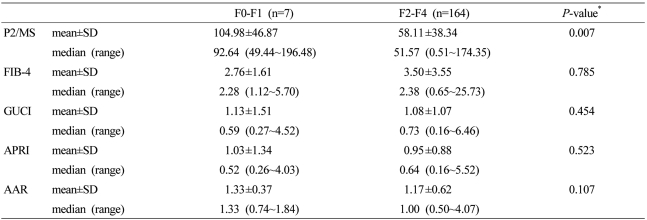
The patient characteristics are summarized in Table 1. One hundred thirty two (77.2%) patients were male, and the mean age was 56.1±10.2 years. One hundred thirty six (79.5%) patients belonged to AJCC stage I and II. Fibrosis stage was determined in all 171 patients: seven patients with METAVIR F1, 31 with F2, 41 with F3, and 92 with F4. Scores for P2/MS ranged from 0.51 to 196.48.
Diagnostic accuracy of P2/MS
The mean values decreased as a function of the fibrosis score, ranging from 104.97±46.87 for F1 cases to 44.20±33.30 for F4 cases. P2/MS scores showed a significant inverse Spearman's correlation with the METAVIR fibrosis score (Fig. 1; Spearman's correlation coefficient = -0.481, P<0.001). However, P2/MS scores didn't show a significant Spearman's correlation with TNM stage (Spearman's correlation coefficient = -0.020, P=0.799).
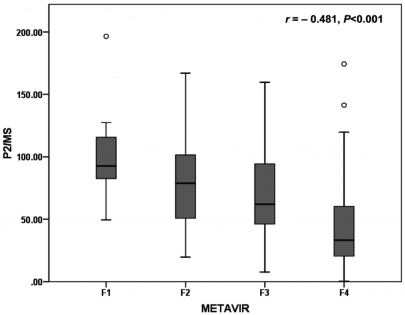
Box plot of P2/MS according to the fibrosis stage by the METAVIR scoring system.24 Boxes represent interquartile ranges, whiskers indicate highest and lowest values, circles represent outliers, and horizontal lines within boxes indicate median values. P2/MS values showed a significant inverse Spearman's correlation according to the METAVIR fibrosis score.
P2/MS, [platelet count (109/L)]2/[monocyte fraction (%)×segmented neutrophil fraction (%)].
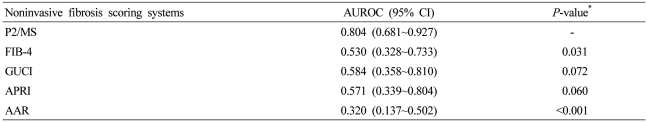
Regarding the fibrosis grade, the AUROC was 0.804 [95% confidence interval (CI), 0.681~0.927; P=0.007] for detecting significant fibrosis (F2-F4), 0.749 (95% CI, 0.669~0.829; P<0.001) for detecting severe fibrosis (F3-F4), and 0.769 (95% CI, 0.698-0.839; P<0.001) for detecting histological cirrhosis (F4) (Fig. 2). The sensitivity, specificity, PLR, and NLR of P2/MS were calculated using previously published cut-off values (Table 2).17,18 At a cut-off value <62, P2/MS detected significant fibrosis with a specificity of 85.7% (95% CI, 42.0~99.2) and a PLR of 4.27 (95% CI, 0.69~26.31); and at a cut-off value >115, P2/MS ruled out significant fibrosis with a sensitivity of 90.2% (95% CI, 84.4~94.1) and a NLR of 0.34 (95% CI, 0.11~1.10). At a cut-off value <30, P2/MS detected histological cirrhosis with a specificity of 92.4% (95% CI, 83.6~96.9) and a PLR of 5.30 (95% CI, 2.36~11.89); and at a cut-off value >83, P2/MS ruled out histological cirrhosis with a sensitivity of 89.1% (95% CI, 80.5~94.4) and a NLR of 0.26 (95% CI, 0.14~0.48).

ROC curves of P2/MS for detection of fibrosis. P2/MS showed significant diagnostic accuracy for detection of fibrosis. The AUROC was 0.804 (P=0.007) for detection of significant fibrosis (F2-F4) (A), 0.749 (P<0.001) for detection of severe fibrosis (F3-F4) (B), and 0.769 (P<0.001) for detection of histological cirrhosis (F4) (C).
ROC, receiver-operating characteristic; P2/MS, [platelet count (109/L)]2/[monocyte fraction (%)×segmented neutrophil fraction (%)]; AUROC, area under the receiver operating characteristic curve.
To avoid the confounding effects of the initial tumor stage and the etiology of HCC, we evaluated the diagnostic accuracy of P2/MS according to HCC stage and the etiology of HCC, which could affect the hematologic data or hepatic fibrosis status. For 119 (69.6%) patients of AJCC stage I, the AUROC of P2/MS was 0.821 (95% CI, 0.731~0.910; P=0.121) for detecting significant fibrosis (F2-F4), 0.724 (95% CI, 0.617~0.832; P=0.001) for detecting severe fibrosis (F3-F4), and 0.721 (95% CI, 0.630~0.812; P<0.001) for detecting histological cirrhosis (F4). Within HBVrelated HCC [160 (93.6%) of all patients], the AUROC of P2/MS was 0.821 (95% CI, 0.7230.918; P=0.029) for detecting significant fibrosis (F2-F4), 0.731 (95% CI, 0.645~0.817; P<0.001) for detecting severe fibrosis (F3-F4), and 0.766 (95% CI, 0.692~0.839; P<0.001) for detecting histological cirrhosis (F4).
Comparison of P2/MS and other noninvasive fibrosis scoring systems
Mean values of noninvasive fibrosis scoring systems were summarized in Table 3. To evaluate the diagnostic value of noninvasive fibrosis scoring systems for significant fibrosis, we compared the AUROC of P2/MS with those of other tests (Table 4). The AUROC was greatest for P2/MS (0.804; 95% CI, 0.681~0.927), then GUCI (0.584; 95% CI, 0.358~0.810) followed by APRI (0.571; 95% CI, 0.339~0.804), FIB-4 (0.530; 95% CI, 0.328~0,733), and AAR (0.320; 95% CI, 0.137~0.502). Furthermore, there was a significant difference between the AUROC for P2/MS and the AUROCs for FIB-4 and AAR. The AUROC of P2/MS showed a tendency to increase compared to those of GUCI and APRI (Fig. 3A). For histological cirrhosis, although there were no statistically significant differences between the AUROC for P2/MS and the AUROCs for other noninvasive fibrosis scoring systems except AAR, the AUROC was greatest for P2/MS (0.769; 95% CI, 0.698~0.839), then GUCI (0.717; 95% CI, 0.639~0.795) followed by FIB-4 (0.704; 95% CI, 0.627~0.782), APRI (0.701; 95% CI, 0.622~0.780), and AAR (0.536; 95% CI, 0.449~0.623) (Fig. 3B, Table 5).
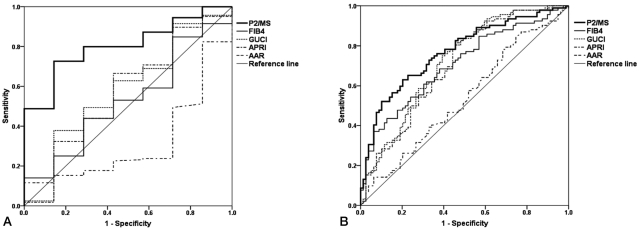
Comparison of ROC of P2/MS and other noninvasive fibrosis scoring systems for diagnosis of significant fibrosis (F2-F4) (A) and histological cirrhosis (F4) (B). For detection of significant fibrosis (F2-F4) and histological cirrhosis (F4), the AUROC was the greatest for P2/MS.
P2/MS, [platelet count (109/L)]2/[monocyte fraction (%)×segmented neutrophil fraction (%)]; GUCI, Goteborg University Cirrhosis Index; APRI, AST to platelet ratio index; AAR, AST to ALT ratio; ROC, receiver-operating characteristic; AUROC, area under the receiver operating characteristic curve.
DISCUSSION
The principal findings of this study relate to the detection of significant hepatic fibrosis of patients with HCC by P2/MS. P2/MS showed an inverse Spearman's correlation with the METAVIR score of patients with HCC and statistically significant diagnostic accuracy for detecting significant fibrosis (METAVIR F2-F4), severe fibrosis (METAVIR F3-F4), and histological cirrhosis (METAVIR F4). Furthermore, among noninvasive fibrosis scoring systems, only P2/MS showed superior diagnostic accuracy to other noninvasive fibrosis scoring systems for detecting significant fibrosis (METAVIR F2-F4) in this study. For histological cirrhosis (METAVIR F4), the diagnostic accuracy was greatest for P2/MS among noninvasive fibrosis scoring systems and there was a statistically significant difference between that of P2/MS and AAR.
The early detection of hepatic fibrosis is important for predicting clinical outcomes of patients with HCC.4 In addition, hepatic fibrosis is a determinant factor for the antiviral therapy strategy in patients with HBV replication and raised ALT levels, or those with high normal ALT levels and who are older than 40 years.25 However, the current gold standard for the diagnosis of hepatic fibrosis is a liver biopsy which has several disadvantages, such as poor compliance, invasiveness, complications, and cost.9 Therefore, noninvasive modalities of evaluating hepatic fibrosis have been proposed to overcome these problems. The two principal categories of noninvasive tests include serum fibrosis panels and hepatic elasticity imaging.23 However, these modalities have not showed sufficient diagnostic efficacy for complete replacement of liver biopsy.18 In the meantime, P2/MS, a new, accurate method, based only on a complete blood cell count, was developed for reflecting significant fibrosis (METAVIR F2-F4) and cirrhosis (METAVIR F4) in virus-related CLD.17 Moreover, this was reconfirmed recently by two external comprehensive and prospective validation studies.18,19 However, a validation study is necessary before applying P2/MS to patients with HCC and our study is the first investigation to examine the diagnostic value of P2/MS in patients with HCC.
In this study, P2/MS detected significant fibrosis (METAVIR F2-F4) at a cut-off value <62 and effectively ruled out significant fibrosis (METAVIR F2-F4) at a cut-off value >115. This was consistent with the result of a P2/MS study by Lee et al.17 However, for the detection of histological cirrhosis, P2/MS detected histological cirrhosis at a cut-off value <30, and effectively ruled out histological cirrhosis at a cut-off value >83. This was not consistent with a P2/MS study by Lee et al,17 but was consistent with the cut-off values proposed by Kim et al.18 Among our study population, chronic hepatitis B (CHB) patients were 93.6% and chronic hepatitis C (CHC) patients are only 6.4%. The study population of the external validation study by Kim et al18 consisted entirely of all CHB patients. In contrast, in P2/MS study by Lee et al,17 46 (22.4%) of 205 patients in the derivation set were CHC patients. The composition of our study population was more similar to the external validation study by Kim et al.18 and for the detection of histological cirrhosis in patients with HCC, cut-off values proposed by Kim et al18 seem to be acceptable.
The AUROC of FIB-4 and GUCI were 0.704 and 0.717 for diagnosis of histological cirrhosis in our study, respectively. These are somewhat inferior to previous reports for the FIB-4 and GUCI where AUROCs of 0.91 and 0.85 have been reported.20,21 The discrepancies seem to originate from the difference in study populations between our study and previous studies. CHB patients were dominant and a few CHC patients were enrolled in our study. In contrast, study populations of previous studies were composed of hepatitis C virus-infected patients only. Recently, it has been suggested that the pathogenesis of liver fibrosis in CHB is different from that of CHC,26 in which the hepatic necroinflammatory activity of CHB is decreased after recurrent hepatitis B flares and HBeAg seroconversion is observed after the development of cirrhosis.27 In contrast, CHC is a progressive disease with persistent inflammation that is related to liver cirrhosis.27 Consequently, CHB has specific mechanisms for liver fibrosis and cirrhosis different from those of CHC.27 Moreover, patients with space-occupying lesions such as HCC might have higher transaminase levels.27 In addition, the fluctuating patterns of transaminase activity in patients with chronic viral hepatitis may be an important limitation for the use of noninvasive fibrosis scoring systems containing AST or ALT.28
The diagnostic accuracy of P2/MS was not increased when analyzed according to tumor stage or the etiology of HCC. That might be related with small case numbers of our study and it made our study prone to type II errors.29 Thus, prospective studies with large number of patients are warranted to evaluate the effect of tumor stage or the etiology of HCC on the diagnostic accuracy of P2/MS.
The AUROC of P2/MS for significant fibrosis (METAVIR F2-F4) and histological cirrhosis (METAVIR F4) were 0.804 and 0.769 in our study, respectively. These relatively lower diagnostic yields compared to the previous studies excluding HCC patients may be related to smaller changes in the platelet count along with monocyte and segmented neutrophil fractions in mild liver fibrosis than in cirrhosis as discussed before.17 Although the platelet count was thought to be a significant predictor of liver fibrosis in patients with CLD, platelet counts could significantly vary over time among patients with CLD.27 Most of the patients with HCC had severe fibrosis (METAVIR F3-F4) or histological cirrhosis (METAVIR F4), and thereafter the paucity of normal to mild liver fibrosis for comparison was a limitation. Although the performance of P2/MS for detecting significant fibrosis (METAVIR F2-F4) in patients with HCC was not satisfactory, this index had greatest efficacy among noninvasive fibrosis scoring systems. However, since our study was a retrospective analysis performed in a single medical center, an independent external validation of P2/MS in patients with HCC by prospective studies is required. Moreover, compared to previous study about APRI or AAR, the case numbers are too small, so further prospective studies are warranted for precise comparison among well known noninvasive fibrosis scoring systems.
In conclusion, P2/MS showed significant diagnostic accuracy for the detection of significant fibrosis (METAVIR F2-F4), severe fibrosis (METAVIR F3-F4), and histological cirrhosis (METAVIR F4) in patients with HCC. Furthermore, P2/MS showed the greatest diagnostic accuracy among noninvasive fibrosis scoring systems in patients with HCC. These findings indicate that P2/MS, which is only based on complete blood cell count and differential cell count results, maintained diagnostic accuracy in patients with HCC. Therefore, P2/MS can be utilized as a noninvasive fibrosis scoring systems reflecting the degree of hepatic fibrosis in patients with HCC as well as ones with viral hepatitis, but without HCC.
Abbreviations
P2/MS
[platelet count (109/L)]2/[monocyte fraction (%)×segmented neutrophil fraction (%)]
CI
confidence interval
CHB
chronic hepatitis B
HCC
hepatocellular carcinoma
HBV
hepatitis B virus
CHC
chronic hepatitis C
BCLC
Barcelona Clinic Liver Cancer
CLD
chronic liver disease
AJCC
American Joint Committee on Cancer
AST
aspratate transaminase
ALT
alanine transaminase
GUCI
Goteborg University Cirrhosis Index
INR
international normalization ratio
APRI
AST to platelet ratio index
AAR
AST to ALT ratio
H&E
hematoxylin and eosin
ROC
receiver operating characteristic
AUROC
area under the ROC curve
PLR
positive likelihood ratio
NLR
negative likelihood ratio