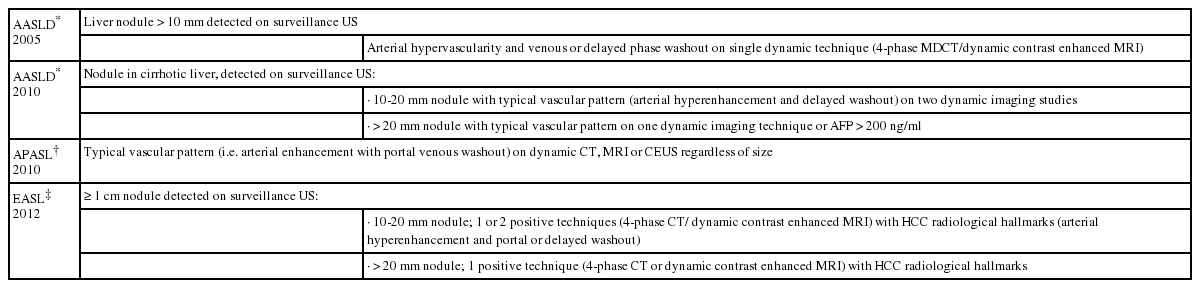
Recent advances in the imaging of hepatocellular carcinoma
Article information
Abstract
The role of imaging is crucial for the surveillance, diagnosis, staging and treatment monitoring of hepatocellular carcinoma (HCC). Over the past few years, considerable technical advances were made in imaging of HCCs. New imaging technology, however, has introduced new challenges in our clinical practice. In this article, the current status of clinical imaging techniques for HCC is addressed. The diagnostic performance of imaging techniques in the context of recent clinical guidelines is also presented.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy.1 The overall incidence of HCC is steadily rising across the world: its overall incidence remains alarmingly high in the developing countries and is increasing in most of the developed countries as well.2 Early diagnosis and accurate assessment of disease extent are crucial for appropriate clinical management of patients with HCC to help support curative treatment and excellent prognosis.3 Of various clinical diagnostic tools, in particular, imaging plays a vital role in surveillance, characterization, staging, and treatment monitoring of HCC in recent clinical guidelines proposed by the Barcelona European Association for the Study of the Liver (EASL) Conference, the Asian Pacific Association for the Study of the Liver (APASL), and the American Association for Study of Liver Diseases (AASLD) (Table 1).4,5,6
It is widely accepted that hepatocarcinogenesis is a multistep process.7 The two key events of the process developing simultaneously are: (1) progressive cellular morphologic and functional changes and (2) sequential changes in the intranodular blood supply including progressive decrease of the portal supply and increase in the number of unpaired arteries.8,9,10
These carcinogenetic changes can be detected and characterized by means of various clinical imaging techniques. For instance, cellular morphologic changes can be depicted using gray scale ultrasonography (US), unenhanced computed tomography (CT), and unenhanced magnetic resonance imaging (MRI) including diffusion-weighted imaging (DWI). On the other hand, the application of contrast agents facilitates the evaluation of functional changes held in a nodule during hepatic carcinogenesis beyond morphologic changes. Contrast-enhanced US using a new US contrast agent, Sonazoid (GE healthcare),11 and enhanced MRI with superparamagnetic iron oxide (SPIO) can depict the presence of Kupffer cells in a nodule.12 Changes in signal intensity on hepatobiliary images of Gd-EOB-DTPA-enhanced MRI correlate with organic anion transporting polypeptides 8 (OATP 8) expression.13 Multiphase CT and MRI with contrast agents provide the information related to hemodynamic changes in a nodule that are currently regarded as the most important diagnostic criteria for HCC in routine clinical practice.4,5,6
Over the past few years, considerable technical advances were made in imaging of HCCs. New imaging technology, however, has introduced new challenges in our clinical practice. We must decide how best to standardize protocols, appropriate imaging protocols for clinical indications, and ensure diagnostic efficacy. In this article, the current status of clinical imaging techniques for HCC is addressed. The diagnostic performance of imaging techniques in the context of recent clinical guidelines is also presented.
Imaging characteristics and diagnosis of HCC
Gray scale US is the most commonly used imaging test for surveillance since it is relatively inexpensive, noninvasive, and well accepted by patients.4,5,6 A systematic review of 14 US studies on the accuracy of US in diagnosing HCC published the sensitivity of 69% and specificity of 97%.14 However, the diagnostic performance of US is significantly affected by multiple factors including the expertise of sonographer, the body habitus of patients, and size and location of lesions.15 US features of HCC are variable and may be indistinguishable from benign nodules.16 HCC usually presents as a discrete nodule with heterogenous echogenicity or a mosaic pattern, and thin hypoechoic peripheral zone which represents the tumor capsule (Fig. 1).17,18 In particular, since gray scale US reflects only nonspecific cellular morphologic changes in a nodule, its use is indicated only for the detection of HCC.4,5,6 With US contrast agents, US can provide vascular and functional information. US contrast agents are coated microbubbles that serve as an acoustic reflector and appear as high echogenecity in the location where contrast agents present.19 Blood-pool US contrast agent such as SonoVue (Bracco Diagnostics) provides detailed real-time hemodynamic information.19,20 However, some recent data demonstrated intrahepatic cholangiocarcionoma could present with vascular patterns similar to HCC. This led a recent revision in the AASLD guideline to exclude contrast-enhanced US from the list of diagnostic techniques that can be used to characterize lesions suspicious for HCC.21,22 A recently introduced US contrast agent, Sonazoid (GE healthcare) is phagocytosed by Kupffer cells of the liver that can be seen during the postvascular phase or Kupffer phase (starting 10 min after the injection) (Fig. 2).11 This contrast agent also provides real-time hemodynamic information as a blood-pool US contrast agent in earlier phase of imaging. The enhancement characteristics of Sonazoid would help expand the role of contrast-enhanced US imaging for the surveillance and diagnosis of HCC.23,24 Accordingly, the APASL guideline included the indication of Sonazoid US imaging for further evaluation of lesions with atypical imaging features.5
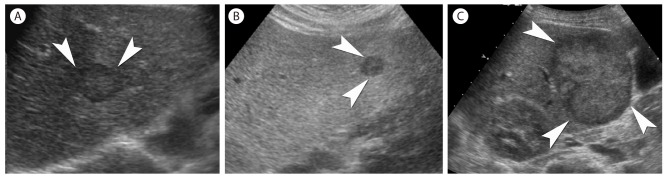
Various gray scale US features of HCCs. (A-C) On gray scale US, HCC (arrowheads) can be seen as a nodule with thin hypoechoic peripheral zone (A), a discrete hypoechoic nodule (B), or a mass with heterogenous echogenicity (C) in comparison to the surrounding hepatic parenchyma.

Contrast-enhanced US images of HCC. (A) On a gray scale US image, HCC is barely visible. (B) An arterial phase image of Sonazoid-enhanced US detects the strong arterial hypervascularity of HCC (arrows). (C) On a Kupffer phase image of Sonazoid-enhanced US, HCC (arrow) appears hypoechoic compared to the enhanced surrounding hepatic parenchyma. Thus, contrast-enhanced US using Sonazoid presents both vascular and functional information of lesions. In addition, it can possibly enhance the visibility of HCCs.
In a common clinical setting, a hepatic nodule detected on surveillance US is further interrogated with contrast-enhanced multiphase CT or MRI to demonstrate the presence of a specific vascular profile (i.e., contrast wash-in during the arterial phase followed by contrast wash-out during the portal or the delayed phase) (Fig. 3).5,6 According to the revised AASLD guidelines, the sensitivity of detecting 1-2 cm HCC was 44% with CT and 44% with MRI, while the specificity was 100% with either CT or MRI.25 The typical vascular pattern of HCC was identified in 65% of nodules by a single technique while maintaining the high specificity.25 Another study, in support of the revised AASLD guidelines, reported that a sequential combination of the two imaging methods, requiring only one to be positive, yielded the sensitivities of 74-89% and the specificities of 91-99%.26 Currently, CT is the most commonly used imaging method for the diagnosis and staging of HCC, because of its wide availability and high temporal and spatial resolution. On the other hand, the strengths of MRI include superb soft-tissue contrast and information on the tissue composition (Fig. 4). According to a meta-analysis, the pooled sensitivity and specificity for the detection of HCC with contrast-enhanced MRI were 81% and 85%, respectively, while those with contrast-enhanced CT were 68% and 93%, respectively.14 Several studies compared contrast-enhanced MRI and CT in the same patient population for the detection of HCC and published that the sensitivities were higher with MRI (61-90%) than with CT (54-78%).27,28,29,30 The differences in the sensitivities between the two imaging modalities were more pronounced in the detection of small HCC nodules of 1-2 cm in diameters (84-85% with MRI vs. 47-68% with CT).27,29
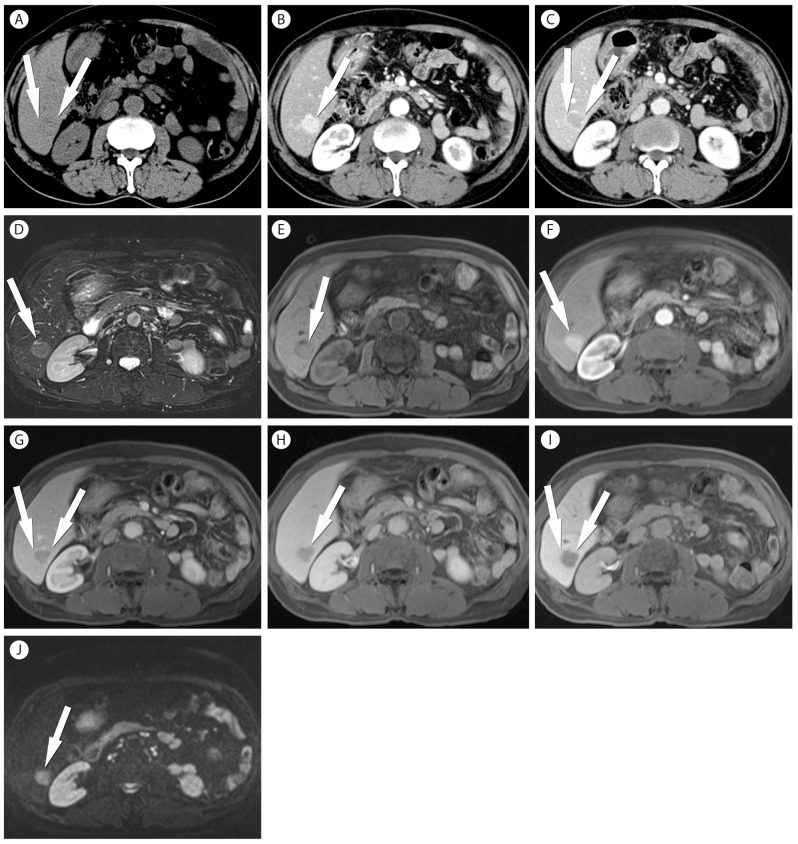
Typical CT and Gd-EOB-DTPA-enhanced MRI features of HCC. (A) An axial noncontrast image shows a subtle low density lesion (arrows) in right lobe of the liver. (B, C) On contrast enhanced CT images, the typical enhancement patterns the lesion (arrows), i.e. arterial hypervascularity (arrow in b) as well as washout on portal phase images (arrows in C) are demonstrated. (D, E) HCC (arrows) looks hyperintense on T2-weighted (D) and hypointense on T1-weighted (E) MRI. (F-H) On dynamic phase images of Gd-EOB-DTPA-enhanced MRI, HCC (arrows) also shows the typical enhancement pattern. (F, arterial phase image; G, portal phase image; H; transitional phase image). (I) A hepatobiliary phase image of of Gd-EOB-DTPA-enhanced MRI depicts a hypointense nodule (arrows) in right lobe of the liver with an increased conspicuity of the lesion in the background of hyperintense hepatic parenchyma. (J) On a DWI, HCC (arrow) appear as a definite hyperintense nodule.

Atypical enhancement pattern of HCC. (A-C) In a male patient with chronic hepatitis B and an elevated level of alpha fetoprotein, the first contrast-enhanced CT fails to detect lesions suspicious of HCCs. On a noncontrast imge (A), a hypodense lesion (arrowheads) is found in left lobe of the liver, while it is not seen on arterial (B) and portal (C) phase images. (D-G) Gd-EOB-DTPA-enhanced MRI at the similar time of the first CT images, also fails to find typical vascular enhancement pattern of the lesion, although the lesion (arrowheads) is seen hyperintense on a T2-weighted image (D). A hepatobiliary phase image (G) demonstrates a hypointense lesion (arrowheads). (E, arterial phase image; F. portal phase image). (H,I) 6-month follow up CT images finally define the typical arterial hypervascularity (arrowheads in h) and washout on a portal phase image (arrowheads in I).
Early HCCs frequently present with atypical contrast enhancement patterns reflecting their immature intranodular vascular changes in histological level.7,31,32 As many as 87% of well-differentiated lesions and 41-62% of lesions smaller than 2 cm may show atypical vascular patterns (Fig. 4).31,32 An important clinical quandary is that these atypical lesions are the main target of the surveillance program because they respond more likely to curative treatments than other typical late-stage lesions.33,34 However, on the basis of the AASLD guidelines that stipulate typical contrast enhancement profiles for the diagnosis of HCC, the sensitivity for detecting early HCCs is rather limited. The APASL guidelines, underscoring the usefulness of Kupffer cell specific imaging such as SPIO-enhanced MRI and Sonazoid-enhanced US, may be more supportive to increase the sensitivity for the diagnosis of HCCs with atypical vascular profiles (Fig. 4).5,6,35
Two recent significant advances in liver MRI are the introduction of hepatocyte-specific contrast agents such as Gd-EOB-DTPA and the application of DWI in routine clinical liver MR examination. Between the two currently available hepatocyte-specific contrast agents, Gd-EOB-DTPA is advantageous over Gd-BOPTA because the hepatocyte uptake percentage (50% of the administered) of Gd-EOB-DTPA is greater than that (5%) of Gd-BOPTA. As a result, the hepatobiliary phase imaging with Gd-EOB-DTPA yields superior hepatic enhancement and requires a time delay (20 minutes) shorter than that with Gd-BOPTA (60-120 minutes).36,37 In this hepatobiliary phase images, malignant tumors such as HCCs are spared from the contrast uptake that occurs in the surrounding liver because of the absent or the hampered function of hepatocytes in malignant tumors.17
The assessment of added diagnostic value of the hepatobiliary phase imaging is currently in the field of active research. Several studies reported that Gd-EOB-DTPA enhanced MR outperformed CT for the diagnosis of HCC.38,39 In particular, Gd-EOB-DTPA was advantageous over CT for small lesions less than 1 cm40 and for the differentiation of small HCCs from hypervascular pseudolesions in patients with chronic liver disease.41,42 Moreover, Gd-EOB-DTPA hepatobiliary phase imaging features can be used as biomarkers to predict microvascular invasion, tumor aggressiveness, and even patient's outcome.43,44,45,46
While Gd-EOB-DTPA-enhanced MRI offers a number of exciting opportunities as a new imaging tool, there remain some challenges in clinical application of Gd-EOB-DTPA-enhanced MRI. First, hypointense nodules that are observed only during the hepatobiliary phase are frequently encountered in routine clinical practice. Yet, the clinical significance of these nodules is unclear. Several studies reported that a considerable proportion (27.6-43.5%) of non-specific hypointense nodules presented at the initial hepatobiliary phase images showed interval changes in their MR signal and morphological characteristics at follow-up MRI.47,48,49,50 Potential factors associated with the interval transformation of these lesions included the size (>15 mm), tumor doubling time (<542 days), the presence of fat, high signal intensity on T1-weighte images, and high signal intensity on DWI.47,48,49,50 The optimal follow-up interval and treatment strategy for these hepatobiliary hypointense nodules are yet to be determined. Second, mass-forming intrahepatic cholangiocarciomas may mimic HCCs on Gd-EOB-DTPA-enhanced MRI (Fig. 5).51 Arterial hypervascularity was reported in 30% of intrahepatic cholangiocarcionomas, especially in small tumors less than 3 cm.52 Furthermore, the characteristic delayed enhancement of cholangiocarcinoma could not be well demonstrated on Gd-EOB-DTPA MRI because the inherently high background hepatocyte uptake may affect the imaging features on the delayed phase images contributed by the extracellular distribution of Gd-EOB-DTPA contrast agent.51 Third, the acquisition of optimal arterial phase images in Gd-EOB-DTPA enhanced MR is more challenging than other conventional extracellular MRI contrast agent.53 This is likely due to the difference in the amount of administered contrast agents: Gd-EOB-DTPA typically administered at 0.1 mL/kg body weight while conventional extracellular contrast agents at 0.2 mL/kg body weight.53 Weak arterial enhancement may negatively affect the conspicuity of detecting hypervascular lesions such as HCC. In addition, there was a report showing that transient tachypnea after administration of Gd-EOB-DTPA possibly decreased the quality of the arterial phase images of Gd-EOB-DTPA-enhanced MR.54

Arterial hypervascular cholangiocarcionoma. (A) In a male patient with chronic hepatitis C, a T2-weighted image finds a mass (arrow) with a lobulated contour in left lobe of the liver. (B) On an arterial phase image of Gd-EOB-DTPA-enhanced MRI, the lesion (arrows) shows strong arterial hypervascularity. (C, D) Transitional phase (C) and hepatobiliary phase (D) images obtained 3 and 20 minutes after the administration of Gd-EOB-DTPA, respectively, depict a hypointense mass (arrows). Given the risk factor of the patient and the enhancement pattern, the primary diagnosis was HCC. However, the lesion was confirmed as cholangiocarcinoma after surgery.
DWI reflects the diffusion process of molecules, mainly water, in tissue and allows us to characterize tissue microstructural changes. A highly cellular tissue such as malignant tumor results in restriction of the apparent diffusion of water molecules and a decrease in the apparent diffusion coefficient (ADC) values.17 As a result, liver tumors including HCCs appear as high signal intensity lesions on DWI in contrast to the low signal intensity of the liver parenchyma.55 Studies reported higher rates of detection of HCCs with added DWI (sensitivity of 84-98%) than multiphasic MRI alone (sensitivity of 76-85%),56,57,58 although the results are still debatable.59 Combination of hepatobiliary phase images of Gd-EOB-DTPA and DWI would improve the diagnostic performance of MRI, especially for nodules with atypical vascular pattern and small size.60,61,62
Guideline for imaging diagnosis of HCC
The interpretation of liver imaging and the radiologic reporting become more complex with new, advanced imaging techniques. In a common clinical setting, patients often undergo multiple imaging studies that are interpreted by multiple radiologists. These added complexities lead to inconsistent interpretations and reporting of radiological studies. As an initiative to address this problem, the American College of Radiology proposed the Liver Imaging-Reporting and Data System (LI-RADS) (Accessed February 2015, from http://www.acr.org/Quality-Safety/Resources/LIRADS/). The goals of the initiative are to reduce variability in lesion interpretation by standardizing report content and structure; improving communication with clinicians; and facilitating decision making, outcome monitoring, performance auditing, quality assurance, and research.63 Five categories that follow the diagnostic thought process are used to stratify individual observations according to the level of concern for HCC.63 LI-RADS diagnostic algorithm is intended only for individuals at increased risk for HCC regardless of presence or absence of previous surveillance US or other imaging. Distinct from AASLD guideline, LI-RADS expanded the "Indeterminate" category into probably benign, intermediate probability of HCC and probably HCC (LI-RADS categories 2, 3 and 4) to minimize false positive interpretations for 1-2 cm nodule detected by CT or MRI. The latest LI-RADS 2014 includes considerations of cholangiocarcinoma, mixed hepatobiliary tumors, infiltrative HCC as well as the use of hepatobiliary MRI contrast agents.64
CONCLUSION
In summary, continued advances and changes are made in imaging studies for the detection and diagnosis of HCC. MRI with hepatocyte-specific contrast agents and DWI is increasingly accepted, in part because of their potentials for improved diagnosis of early HCCs. In comparison, SPIO-enhanced MRIs on decline because of limited availability of SPIO contrast agents and restricted clinical applicability. Double contrast MR and CT hepatic arteriorgraphy and arterioportography are less commonly used, given their demanding technical complexity. We should keep in mind that the current guidelines for the clinical applicability and appropriateness of imaging diagnosis of HCC, will be constantly refined and updated with on-going advances in imaging techniques and supportive data from clinical validation and research studies.
Acknowledgements
This study was supported by Grant #2012R1A1A1012731 by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Korea.
Notes
Conflicts of Interest: The authors have no conflicts to disclose.
Abbreviations
AASLD
american association for study of liver diseases
ADC
apparent-diffusion coefficient
APASL
asian pacific association for the study of the liver
CT
computed tomography
DWI
diffusion-weighted imaging
EASL
european Association for the study of the Liver
HCC
hepatocellular carcinoma
LI-RADS
liver imaging-reporting and data system
MRI
magnetic resonance imaging
OATP
organic anion transporting polypeptides
SPIO
super-paramagnetic iron oxide
US
ultrasonography