Hepatic venous pressure gradient: clinical use in chronic liver disease
Article information
Abstract
Portal hypertension is a severe consequence of chronic liver diseases and is responsible for the main clinical complications of liver cirrhosis. Hepatic venous pressure gradient (HVPG) measurement is the best available method to evaluate the presence and severity of portal hypertension. Clinically significant portal hypertension is defined as an increase in HVPG to >10 mmHg. In this condition, the complications of portal hypertension might begin to appear. HVPG measurement is increasingly used in the clinical fields, and the HVPG is a robust surrogate marker in many clinical applications such as diagnosis, risk stratification, identification of patients with hepatocellular carcinoma who are candidates for liver resection, monitoring of the efficacy of medical treatment, and assessment of progression of portal hypertension. Patients who had a reduction in HVPG of ≥20% or to ≤12 mmHg in response to drug therapy are defined as responders. Responders have a markedly decreased risk of bleeding/rebleeding, ascites, and spontaneous bacterial peritonitis, which results in improved survival. This review provides clinical use of HVPG measurement in the field of liver disease.
INTRODUCTION
Portal hypertension is a clinical syndrome defined by increased portal venous pressure gradient above 5 mmHg due to raised pre-, intra-, or post-hepatic resistance.1 In liver cirrhosis (LC), portal hypertension develops in a case with fibrotic change in sinusoidal liver architecture and is a severe complication of chronic liver disease that severely affects mortality.2 Portal hypertension may lead major complications of LC, including variceal bleeding, ascites, or hepatic encephalopathy.3
Direct measurement of portal pressure is highly invasive and no longer performed. In 1951, Myers and Taylor first used wedged hepatic venous pressure (WHVP), which estimates portal venous pressure by occlusive hepatic vein catheterization.4 Currently, a safe, reproducible and less invasive technique to measure the HVPG has been developed. HVPG represents the gradient between the portal vein and the hepatic vein. For many years, measuring hepatic venous pressures, both the free hepatic venous pressure (FHVP) and the WHVP, either with a wedge catheter or a balloon catheter, has been the standard approach for estimating portal venous pressure.4,5,6
Nowadays, the most well documented marker for portal venous pressure is HVPG measurement. There are emerging data on the ability of HVPG to predict overall liver-related outcomes including risk for variceal hemorrhage.7,8 As such, HVPG was considered the best surrogate prognostic marker of LC and increased HVPG correlated with hepatocellular carcinoma (HCC) risk.9 It has been proposed that serial HVPG measurement could assess fibrosis or cirrhosis despite etiology.10,11 Taken together, HVPG measurement can be used in the diagnosis of liver fibrosis, risk stratification, identification of patients with hepatocellular carcinoma who are candidates for liver resection, monitoring of the efficacy of medical treatment, and assessment of progression of portal hypertension.5,8,12,13,14,15
This review presents the available data in the literature outlining the hemodynamic stage of liver fibrosis, methods for HVPG measurement, complications of HVPG measurement, and finally clinical applications of HVPG.
Hemodynamic stage of chronic liver disease
Liver biopsy is currently the standard for the assessment of hepatic fibrosis and is employed for prognostication and decision making processes. Histologically, LC is defined as a diffuse process in which the normal anatomical lobules are replaced by architecturally abnormal nodules separated by fibrous tissue.16 Though there is progressive and continuous liver injury in chronic liver disease, the normal liver has only a small amount of fibrous tissue in relation to its size. Therefore, liver biopsy may not fully represent the state of liver or the histologic features of cirrhosis have not been traditionally linked to clinical outcomes.
HVPG measurement has evolved from being mainly used with diagnostic purposes to be considered a useful tool to assess the severity and prognosis of chronic liver disease and LC, including the risk of the complications such as varices bleeding, ascites, encephalopathy, or hepatorenal syndrome.17
As recently highlighted,18 there is a pressing need for a new classification system for cirrhosis that integrates histologic, clinical, hemodynamic and biologic features. This new classification is necessary for overcoming the limitation of prematurely concluding cirrhosis as an end-stage of chronic liver diseases. This new system classified the distinction between compensation and decompensation which is mainly defined by clinical outcome (Table 1).7,19
Ripoll et al20 found that HVPG greater than 10 mmHg predicts the likelihood of developing decompensation. The cirrhotic stage (METAVIR F4) is broadly classified into 2 stages: compensated and decompensated, with clinical decompensation being defined by the development of ascites, variceal hemorrhage, encephalopathy, and jaundice. HVPG score >6 mmHg indicates portal hypertension and HVPG >10 mmHg represents clinically significant portal hypertension. Within the compensated stage, patients can be sub-classified into those without varices (stage 1) and those with varices (stage 2).18 It is considered moderate or subclinical when HVPG >6 mmHg (Stage 1 compensated liver cirrhosis).18 It becomes 'clinically significant' when >10 mmHg (Stage 2 compensated LC), and 'severe' when >12 mmHg (Stage 3 decompensated LC; Stage 4 decompensated LC) (Table 1).7,8
At the non-cirrhotic stage of chronic liver disease (METAVIR F1-F3), there is no histological or clinical evidence of LC, and portal pressure is within normal range (1-5 mmHg). Compensated LC is classified based on the absence or the presence of varices and the risk of death is low.7 Patients with an HVPG ≤10 mmHg reveal a 90% chance of remaining free of varices and decompensation.21 Patients with an HVPG >10 mmHg present clinical decompensation (22% at 2 years). In patients with decompensated LC, an HVPG >16 mmHg or HVPG >20 mmHg are an important predictor of poor outcome (Table 1).22,23,24
Methods for HVPG measurement
In the normal liver, inter-connected sinusoidal network partially dissipates the pressure backup from the wedged catheter, and the WHVP is slightly lower than directly-measured portal pressure. In LC, the inter-sinusoidal communications are blocked by fibrous tissue, dissipation of pressure in the wedged vessels is insignificant and the WHVP accurately estimates portal pressure.25 Therefore, it is important to note that the use of a balloon catheter instead of a straight one allows occluding a large hepatic vein branch at the lobar and sub-lobar level.
Patients who have severe cardiopulmonary disease, encephalopathy, or hypersensitivity to contrast dye are contraindicated in HVPG measurement. Permission and fasting for 8 hours are needed for the preparation of HVPG measurement. Equipment such as electrocardiography monitor, O2 saturation monitor, pressure recorder, pressure transducer, fluoroscopy, and ultrasonography (option) are needed. In addition, 6 French balloon catheter, puncture needle, vascular introducer, contrast dye, and local anesthetic are required for the proper measurement (Fig. 1). There are several operating manual for the adequate calibration and recording. These are described in Table 2.
Antecubital, femoral, or right jugular veins are possible routes for insertion of catheter in HVPG measurement. In case of right jugular vein insertion, a 6 French balloon catheter is placed in the right hepatic vein through a right jugular vein puncture for measurement of the FHVP. The WHVP is measured by inflation of the balloon catheter at the right hepatic vein. Subsequently, the HVPG is determined by subtracting the FHVP from the WHVP (Fig. 2).
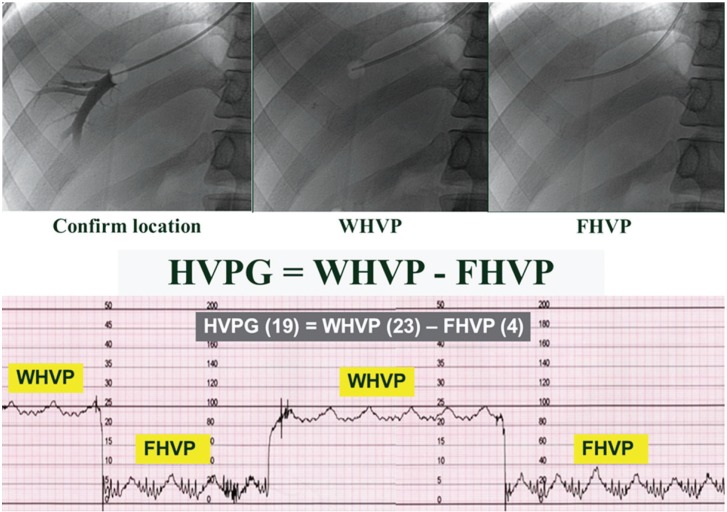
Method for HVPG measurement. HVPG, hepatic venous pressure gradient; WHVP, wedged hepatic venous pressure; FHVP, free hepatic venous pressure.
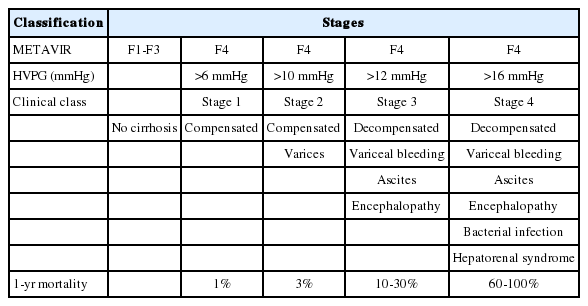
Methods for accurate HVPG measurement are described Table 3.26 For example, the procedure allowed at least 1 minute for WHVP and 15 seconds for FHVP stabilization. The average was taken out of three separate readings. In cases with the presence of shunt, the measurement was taken at a different location to minimize the error (Fig. 3).27
Complications of HVPG measurement
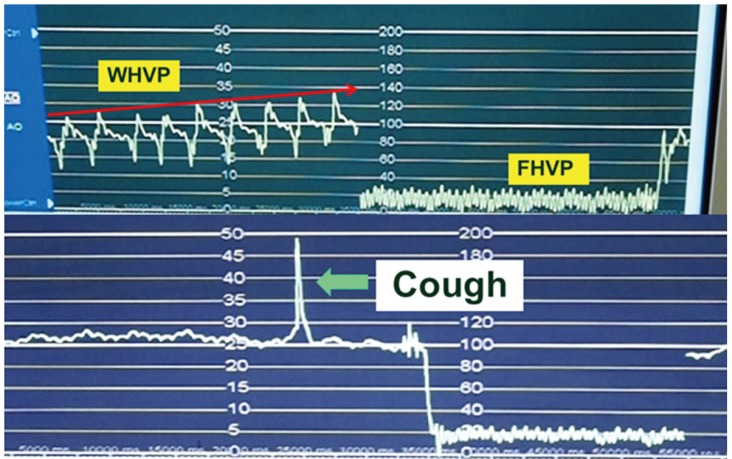
Only minor complications such as mainly transient cardiac arrhythmias, local pain, or vagal reaction have been reported and these occur infrequently (< 1% of patients) (Fig. 4). Until now, no deaths have occurred. HVPG measurements can be performed in 10 minutes with trans-jugular liver biopsy through the same route. Despite its advantages such as safety, feasibility, and reproducibility, the technique is invasive. In addition, HVPG procedure shows low acceptance rate among patients with chronic liver disease and requires technical expertise typically found at tertiary medical centers.28,29
Clinical applications of HVPG
Predicting liver fibrosis
In the diagnosis of stage 1 compensated LC, the sensitivity and specificity of HVPG for predicting stage 1 compensated LC were 78% and 81% in 6 mmHg of HVPG, respectively.14 Kumar et al30 also reported a positive correlation between HVPG and fibrosis score. The AUROC (Area under Receiver Operating Characteristic) of HVPG for predicting advanced fibrosis was 0.906. An HVPG value above 13.0 mmHg had 79% sensitivity and 89% specificity for predicting advanced fibrosis histologically.30 Patients with an HVPG <10 mmHg have a 90% probability of not developing LC.10,20 It was suggested that HVPG (AUROC, 0.85; sensitivity, 80%; specificity, 77%) was a superior diagnostic modality over serologic biomarkers in prediction of advanced fibrosis in chronic viral hepatitis.31 In another study, HVPG was associated with complications associated with portal hypertension, HCC, liver transplantation, and survival.18,32
In the study with post-liver transplant patients, there was a good correlation between liver stiffness measurement (LSM) and HVPG measurements in the overall population.33 In another study, HVPG predicted clinical decompensation in patients with compensated LC. Patients with an HVPG <10 mmHg were found to have a 90% probability of not developing clinical decompensation in a median follow-up of 4 years.20
The most promising of the non-invasive tools to monitor fibrosis progression and associated portal hypertension is LSM by transient elastography.34 The correlation between liver stiffness and HVPG is excellent in patients with HVPG values below 10 mmHg.33 The AUROC for prediction of HVPG 10-12 mmHg ranges from 0.76 to 0.99 with a cutoff of 13.6 to 34.9 kPa.33,35 HVPG >6 mmHg and HVPG >10 mmHg were predicted by 8.7 kPa and 21 kPa cutoff, respectively.36
Predicting outcome of acute variceal bleeding
In patients with acute variceal bleeding, the HVPG measurement provides prognostic information and therapeutic efficacy on the evolution of the bleeding episode. Most studies described that patients with variceal bleeding almost have an HVPG of >12 mmHg.37,38 Short-term prognosis in alcoholic cirrhosis with variceal bleeding was related with porto-hepatic gradient measured within 48 hours of admission.39,40 In other study, an initial HVPG of >20 mmHg was associated with a significantly longer hospital stay, greater transfusion requirements, and worse survival (1-year mortality: 64% vs. 20%, P<0.002).24 Another study suggested that a HVPG value of 11 mmHg is predictive of first variceal hemorrhage with a sensitivity of 92.4% and a specificity of 27.7%.41 Albrades et al39 suggested that HVPG >20 mmHg independently predicted short-term prognosis in patients with acute variceal bleeding treated with a standard vasoactive, antibiotic and endoscopic regimen. Child-Pugh score, systolic blood pressure at admission, and etiology of cirrhosis are also related with short-term prognosis in patients with acute variceal bleeding.
The early effects of endoscopic injection sclerotherapy (EIS) and endoscopic band ligation (EBL) on HVPG during acute bleeding have also been investigated.42 EIS was related with a sustained increase in HVPG compared with EVL. In a study with 50 cirrhotic patients, HVPG was measured before and immediately after endoscopic treatment (EBL and EIS) and every 24 hours, for a 5-day period. In the EBL and EIS groups, a significant increase (18.1 mmHg to 20.7 mmHg and 18.1 mmHg to 21.5 mmHg, P<0.0001) was observed in mean portal pressure immediately after treatment compared with pre-treatment. However, in the EBL group, HVPG returned to baseline values within 48 hours after treatment, while in the EIS group it remained high during the 5-day study period. Thus, during acute variceal bleeding EIS was associated with a sustained increase in HVPG. However, precious mechanism is not clear.
Predicting effectiveness of beta blocker prophylaxis
The yearly incidence of variceal bleeding in cirrhosis patients is estimated at 4%, but this risk increases to 15% according to the size of varices.43 In the aspect of hemodynamic parameter, HVPG ≥10 mmHg is an excellent predictor of the development of varices.21 The haemodynamic response to pharmacological therapy for primary prophylaxis of variceal bleeding has only been evaluated in a few studies, because there is a low bleeding rate and as nonselective beta blockers are effective in primary prophylaxis.
In one study, HVPG was measured in 49 cirrhotic patients with varices at risk of first bleeding, continued therapy with beta-blocker or beta-blocker with isosorbide mononitrate.44 The probability of bleeding at 3 years was significantly higher in poor responders than in good responders (P=0.0008). In a cohort study,45 the usefulness of a strategy in which EBL was used to treat 135 patients was assessed. These patients achieved protection from variceal bleeding comparable to that of good responders to beta-blockers.
Recent meta-analysis suggested that a reduction of HVPG below 12 mmHg or at least 20% from baseline reduced the risk of rebleeding and death.46,47 Pharmacologic therapy has also been used in the prevention of rebleeding in patients with varices. The likelihood of rebleeding in untreated patients is 55-67%. Use of pharmacologic or endoscopic therapy or transjugular intrahepatic portosystemic shunt or other shunts all reduce the risk of bleeding.48,49 The likelihood of a failure to have a hemodynamic response varies from 45 to 63%.50
Other studies have evaluated the use of acute HVPG response to beta-blocker as an alternative target instead of the reduction of HVPG at a repeat measurement in variceal bleeding prophylaxis.51 Gonzalez et al52 evaluated the haemodynamic response guided therapy for prevention of variceal rebleeding in 50 patients over a follow-up of 22 months. Variceal rebleeding occurred in 22% of all patients, but only in 12% of patients whose haemodynamic response was assessed.
Another study evaluated that EBL for the secondary prophylaxis of variceal rebleeding, combination medical therapy guided by HVPG monitoring was more effective than EBL for the secondary prophylaxis of variceal rebleeding and all non-responders would rebleed.53 On the other hand, HVPG has been shown to predict the outcome of the bleeding such as rebleeding and survival.23,24,54
Predicting postoperative outcomes in hepatocellular carcinoma
Preoperative portal pressure is an important predictor of hepatic decompensation in patients with cirrhosis after resection for HCC. Bruix et al55 evaluated that only HVPG was significantly associated with unresolved decompensation within 3 months after surgery (P=0.0001, odds ratio: 1.90). Another study suggested that high portal vein pressure was associated with poor long-term outcome after liver resection for HCC.56 Kim et al12 documented that in decompensated alcoholic cirrhosis, HVPG may be a useful predictive factor for the development of HCC and low serum sodium.
Other applications
HVPG measurement has been evaluated in many prognostic studies, especially in alcoholic cirrhotics.5 HVPG determination is a valuable tool for follow-up in virus recurrence after transplantation.57 Boleslawski et al58 suggested that increased HVPG was associated with post-operative liver dysfunction and mortality after liver resection in HCC and LC, therefore, preoperative HVPG measurement should be measured routinely in these patients. Suk et al59 suggested that initial and follow-up HVPG were necessary to predict survival in decompensated LC with AUROC of 0.843 and 0.864, respectively. In another study, HVPG and albumin were found to be independent predictors of clinical decompensation in patients with compensated virus-related cirrhosis.60
In patients with cirrhosis due to HBeAg-negative chronic B viral hepatitis, lamivudine monotherapy reduced HVPG especially in the presence of virologic suppression and biochemical remission.61 Suk et al59 reported that the efficacy of HVPG in predicting mortality was excellent compared to model for end-stage liver disease (MELD) or MELD-Na. On the other hand, Park et al62 suggested that The MELD-Na is the most predictive for 12-month survival in patients with decompensated cirrhosis. The addition of the HVPG to the MELD or the MELD-Na score does not appear to improve the prognostic accuracy of the MELD or the MELD-Na score significantly.62 Therefore, further well-designed prospective studies are need in the future.
Aside from confirmation of adequate propranolol dosing, HVPG may be needed to predict patient survival with decompensated liver cirrhosis.59 Monitoring the change induced by the treatment of portal hypertension on HVPG, provides strong prognostic information.47 Therefore, in alcoholic LC, follow up using HVPG will help to evaluate the prognosis. In the study with 213 patients followed up for a period of 51.1 months, HVPG was important predictor of decompensation.20
It will be important to establish if a reduction in HVPG follows an improvement in liver function. If there is a survival advantage in lowering HVPG, then propranolol could be advocated for all cirrhotic patients.63
CONCLUSIONS
HVPG measurement is safe, simple, and reproducible method to measure portal pressure. The modern paradigm considers cirrhosis as a dynamic and potentially reversible disease. It consists of two different entities, compensated and decompensated LC. Now, there are many stages in chronic liver disease. Of these stages, HVPG was more predictive of clinical decompensation of cirrhosis than histological fibrosis staging. The information obtained from HVPG may be predictive of new or recurrent bleeding and potentially can help in determining whether or not pharmacologic therapy is effective. The HVPG is the best surrogate marker in portal hypertension and should be measured in every trial involving pharmacologic therapy. In addition, patients with cirrhosis, the HVPG can predict the development of varices, ascites, encephalopathy, or other complications. A reduction in the HVPG is related to a reduction in the incidence of varices and variceal hemorrhage. Therefore HVPG measurement, besides monitoring hemodynamic effects, will mainly assess the all fields of chronic liver diseases.
Acknowledgement
This research was partly supported by Jeil Pharmaceutical CO., LTD. and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2010-0021482).
Notes
The author has no conflicts to disclose.
Abbreviations
AUROC
Area under receiver operating characteristic
EBL
endoscopic band ligation
EIS
endoscopic injection sclerotherapy
FHVP
free hepatic venous pressure
HCC
hepatocellular carcinoma
HVPG
hepatic venous pressure gradient
LC
liver cirrhosis
LSM
liver stiffness measurement
MELD
model for end-stage liver disease
WHVP
wedged hepatic venous pressure