Reactive lymphoid hyperplasia of the liver
Article information
INTRODUCTION
Reactive lymphoid hyperplasia (RLH) is a rare benign condition that forms a mass-like lesion characterized by proliferation of non-neoplastic lymphocytes forming follicles and germinal centers. RLH is thought to represent a reactive immunological response, and some cases may arise in association with malignancy and hepatitis.1 Radiologically, RLH of the liver may mimic a primary or metastatic hepatic malignancy. Therefore, it is very difficult to diagnose correctly without pathological examination. In this issue, we present a case of hepatic RLH and discuss the histopathologic findings with a review of the literatures.
CASE SUMMARY
A 74-year-old woman was referred to our hospital for further evaluation of an intrahepatic bile duct dilatation detected by abdominal ultrasonography (US). She had a history of common bile duct cancer (adenosquamous carcinoma, pT1N0M0), which was resected 4 years ago in our hospital. Physical examination showed intermittent abdominal pain. Blood cell counts and chemical findings, including liver enzymes, were within normal limits. Hepatitis B virus surface antigen was positive and the levels of tumor markers, including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and alpha-fetoprotein (AFP) were all within normal ranges. Abdominal US showed intrahepatic bile duct dilatation of the left lobe. Computed tomography (CT) showed bile duct dilatation with mild ductal wall thickening and enhancement of the left intrahepatic bile ducts (IHBD) (Fig. 1A) and about 1.5 cm sized ill-defined mass like lesion at hepatic segment 2, proximal to the dilated left intrahepatic bile ducts (Fig. 1B). Also, an enlarged sub-hepatic lymph node was detected. Magnetic resonance imaging (MRI) demonstrated multiple IHBD stones that showed a heterogeneous high-intensity on T1-weighted images and low-intensity on T2-weighted images in segment 2. Since these findings suggested cholangitis combined with IHBD stones, the patient underwent left lateral sectionectomy of the liver.

(A) Axial contrast enhanced CT obtained in the arterial phase shows bile duct dilatation with ductal wall thickening and enhancement of the left intrahepatic bile ducts. (B) The CT scan demonstrates about 1.5 cm sized ill-defined mass-like lesion at hepatic segment 2, proximal to the dilated left intrahepatic bile ducts (arrow).
PATHOLOGIC FINDINGS
Macroscopically, the resected lateral segment of the liver had a smooth surface and appeared normal. On the cut section, there were some dilated IHBD without obvious mass-like lesion (Fig. 2A). Microscopically, the lesion exhibited an infiltration of the lymphoid cells with follicles formation and hyalinized interfollicular spaces (Fig. 2B). The lymphoid follicles varied in size and shape with germinal centers composed of small or large lymphoid cells and tingible body macrophages (Fig. 2C). Interfollicular areas were composed of small mature lymphocytes without cytologic atypia. No evidence of recurrent cholagiocarcinoma was observed. Lymphoid cells positive for B cell marker were distributed mainly in the germinal centers, while those positive for T cell marker were observed around the germinal centers (Fig. 2D). Germinal centers were negative for bcl-2. On the basis of the histological and immunohistochemical findings, a final diagnosis of RLH of the liver was made. Post-operatively, no obvious recurrence of the liver nodule was detected during the 3 months medical follow-up.

(A) The cut surface reveals some dilated intrahepatic bile ducts without obvious mass-like lesion. (B) Histological examination shows polymorphous lymphoplasmacytic infiltration with various-sized and shaped lymphoid follicles. (C) The germinal centers of the lymphoid follicles are composed of small or large lymphoid cells and tingible body macrophages (arrows). (D) Immunohistochemical staining reveals CD20 (B cell marker, left) and CD3 (T cell marker, right) to be polyclonal.
DISCUSSION
RLH of the liver is an unusual benign lymphoproliferative lesion, first reported by Snover et al in 1981.2 Since then, 41 cases of RLH in the liver have been reported in the English literature,1 including 2 cases of Korea.3,4 It is characterized by formation of follicles with polymorphic and polyclonal cell population and active germinal centers. RLH can occur in various organs such as skin, orbit, thyroid, breast, lung and gastrointestinal tract,5-7 but it is rare in the liver. Diagnoses of RLH have become frequent during the past two decades due to the use of advanced various imaging modalities and increased population of health screening. Among 42 reported RLH cases, 37 cases are female, while the other 5 cases are male (male-female ratio, 1:7.4), with the mean age of 57 years (range: 15-85 years). The size of the lesion is less than 40 mm in 38 cases and the other 4 cases are more than 40 mm in the greatest dimension (44.4 mm in average; range: 15-105 mm). Most hepatic RLH are single (n=36), but may also be multiple (n=6).
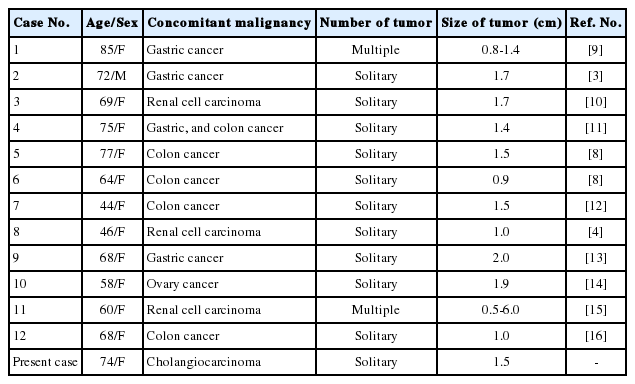
The etiology of RLH is still unclear. In previous reviews, RLH is thought to be associated with an immune-mediated reactive phenomenon. Because one-third of the reported cases had various autoimmune disorders, such as Sjögren's syndrome, CREST syndrome (the limited cutaneous form of systemic scleroderma), autoimmune thyroiditis, primary biliary cirrhosis and primary immunodeficiency.1 Another one-third of the cases had a history of malignancies in various organs, as in our case (Table 1).3,4,8-16 Therefore, it can be considered that the pathogenesis of RLH of the liver in patients with malignant tumor may be related to an immunologic abnormality that is caused by the malignant tumor itself or previous surgery for the tumors.
Pre-operative diagnosis of hepatic RLH is extremely difficult by using imaging studies alone. RLH of the liver may mimic a primary or metastatic hepatic malignancy radiologically, especially in patients with chronic hepatitis or internal malignancies. Fifteen cases out of 41 reported cases before the operation of hepatic RLH had most frequently been misdiagnosed as hepatocellular carcinoma (HCC).1 Some authors described subtle differences in radiological findings between hepatic RLH and HCC. They have reported that hepatic RLH becomes unclear in the delayed phase on MRI scan with injection of contrast agents; while HCC is more hypodense in the delayed phase.1 Therefore, understanding of characteristic imaging of hepatic RLH will better direct the diagnosis.
The definite diagnosis of hepatic RLH is difficult not only radiologically, but also histologically. Histologically, hepatic RLH must be differentiated from low-grade malignant lymphoma, especially the mucosa-associated lymphoid tissue (MALT) type. MALT lymphoma is a low-grade malignant tumor; therefore, it should be distinguished from the hepatic RLH. Both hepatic RLH and MALT lymphoma exhibit a lymphoid infiltrate. It has been reported that lymphoepithelial lesions and cellular atypia with infiltration along the hepatic sinusoid or portal tract are important for diagnosis of MALT lymphoma.15 In hepatic RLH, the lymphoid follicles vary in size with active germinal centers, and neither lymphoepithelial lesion nor lymphocytic atypia are observed. However, it is difficult to distinguish hepatic RLH from MALT lymphoma by conventional histologic evaluation alone. Immunohistochemical and molecular genetic studies are essential to their differentiation. In hepatic RLH, no evidence of monoclonality is observed by immunohistochemistry for B and T cell markers, in situ hybridization for κ and λ light chains, and polymerase chain reaction analysis of immunoglobulin heavy chains or T cell receptor β and γ gene rearrangements, while MALT lymphoma shows a monoclonal population. Usually, pre-operative needle biopsy is not recommended, but it may be useful to differentiate lymphoproliferative lesions from epithelial malignancies, such as HCC or metastatic carcinoma. There have been some reported cases that transformed into malignant lymphoma from RLH in the stomach and lung.17,18 However, there is no reported case that transformed into malignant lymphoma, which had been initially diagnosed as RLH of the liver up to now. Although most patients with RLH of the liver show benign clinical course, complete surgical removal and careful histologic examination are recommended for pathologic confirmation.
Notes
The authors have no conflicts to disclose.
Abbreviations
AFP
alpha-fetoprotein
CA19-9
carbohydrate antigen 19-9
CEA
carcinoembryonic antigen
CT
computed tomography
HCC
hepatocellular carcinoma
IHBD
intrahepatic bile duct
MR
magnetic resonance
RLH
reactive lymphoid hyperplasia
US
ultrasonography