HBsAg level and clinical course in patients with chronic hepatitis B treated with nucleoside analogue: five years of follow-up data
Article information
Abstract
Background/Aims
Quantification of the hepatitis B surface antigen (HBsAg) is increasingly used to determine the treatment response in patients with chronic hepatitis B (CHB). However, there are limited data about the clinical implications of Quantification of HBsAg long-term nucleoside analogue treatment for CHB. We investigated the clinical correlation between HBsAg level and clinical course in patients with CHB who are treated long-term with nucleoside analogues.
Methods
Patients with CHB who started lamivudine or entecavir monotherapy before June 2007 were enrolled. HBsAg was quantified at baseline, at 6 months, and at 1, 2, 3, 4, and 5 years of treatment. We compared data between the groups according to the presence or absence of a virological response (VR) and resistance.
Results
Forty-eight patients were analyzed. There was no definite reduction in HBsAg level during the early period of treatment; differences in HBsAg levels between baseline and each time point were significant only at 5 years (P=0.028). In a subgroup analysis, this difference was significant only in non-resistant patients at 5 years (P=0.041).
Conclusions
There was no definite decrease in the HBsAg level during the early period of nucleoside analogue treatment, with long-term treatment being required to observe a significant reduction.
INTRODUCTION
Quantification of the hepatitis B surface antigen (HBsAg) is increasingly used to determine the treatment response in patients with chronic hepatitis B (CHB). Measuring serum HBsAg concentration during therapy may help to identify sustained responders to pegylated interferon more reliably than that of serum hepatitis B virus (HBV) DNA.1 HBsAg kinetics differ during interferon and nucleoside analogue treatment.2 HBsAg levels remain unchanged during lamivudine (LMV) therapy, whereas recent studies reported an association between a decrease in serum HBsAg and viral suppression after entecavir (ETV) therapy.3-5 However, these studies were performed only after short-term nucleoside analogue treatment. Therefore, evaluations of the clinical implications of long-term treatment in patients with CHB are limited.
The aim of this study was to investigate the clinical correlations between HBsAg level and clinical course in patients with CHB with long-term nucleoside analogues treatment.
METHODS
Patients
We retrospectively reviewed the medical records of patients with CHB who started LMV or ETV monotherapy as first-line treatment before June 2007 at Konkuk University Medical Center, Seoul, South Korea. The final follow-up was completed in December 2012. Patients were censored in cases of liver-related death or unrelated death, loss to follow-up, liver transplantation, or a change to another treatment protocol.
A clinical diagnosis of liver cirrhosis (LC) was based on image findings, using methods such as abdominal ultrasonography, computed tomography or magnetic resonance imaging, together with compatible clinical features such as esophageal varices or thrombocytopenia.
All patients gave written informed consent for the CHB treatment and storage of remnant serum samples. Approval for this study was obtained from the Institutional Review Board of Konkuk University Medical Center.
Measurement of HBsAg levels and response assessment
Serial serum samples were collected from each patient at the time of initiating each antiviral agent, and every 3 months during the treatment and stored at -80℃.
We measured HBsAg levels in stored serum samples at baseline, 6 months, and at 1, 2, 3, 4, and 5 years of treatment using a chemiluminescent microparticle immunoassay (Architect HBsAg QT, Abbott Diagnostics, Chicago, IL, USA). The measurable range of this assay is 0.05-250 IU/mL, and HBsAg was quantified at a 1:500 dilution according to the manufacturers recommendations. Samples with HBsAg levels above or below this range required a lower or higher dilution to bring them within the measurable range.
We also assessed HBV DNA levels using real time polymerase chain reaction (PCR) (Cobas Amplicor PCR, Roche Molecular Systems, Inc., Branchburg, NJ, USA; lower limit of detection, 20 IU/mL). Serum were also assessed. alanine aminotransferase (ALT), bilirubin, HBeAg and anti-HBeAg.
Treatment response was defined as follows:
Virological response (VR): undetectable HBV DNA by real time PCR
Virological breakthrough (VBT): increase in HBV DNA by >1 log10 IU/mL above the nadir at the treatment 2 occasional examination
LMV resistance was detected after VBT using a restriction fragment mass polymorphism (RFMP) method, as described previously.6
Statistical analysis
Continuous variables are expressed as median and range, and categorical variables are expressed as numbers and percentiles. The correlation between HBsAg level and clinical course was analyzed according to VR within 5 years with or without resistance to the antiviral agent. Comparisons between groups were performed using the chi-square test or Fisher's exact test for categorical variables and the Mann-Whitney U-test for continuous variables. Friedman tests were used for the statistical evaluation of the baseline and follow-up HBsAg level during the treatment period. Comparisons between baseline and each time points were performed using Wilcoxon signed-rank test. A p-value < 0.05 was considered significant. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
RESULTS
Baseline characteristics
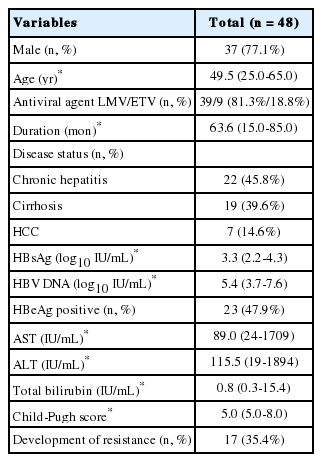
Fourty-eight patients (37 [77.1%] males; median age, 49.5 years) were included in the study. The antiviral agents used were LMV (39, 81.3%) and ETV (9, 18.8%). Median duration of treatment with initial nucleoside analogue was 63.6 months. Underlying disease status was chronic hepatitis in 22 patients (45.8%), LC in 19 (39.6%) and hepatocellular carcinoma (HCC) in seven (14.6%). The baseline median HBsAg and HBV DNA levels were 3.3 log10 IU/mL and 5.4 log10 IU/mL, HBeAg was positive in 23 patients (47.9%). The median aspartate aminotransferase (AST), ALT and total bilirubin levels were 89.0 IU/mL, 115.5 IU/mL, and 0.8 IU/mL. The median Child-Pugh score was 5.0, and resistance developed in 17 patients (35.4%) (Table 1).
Comparison of HBsAg level reduction according to virological response
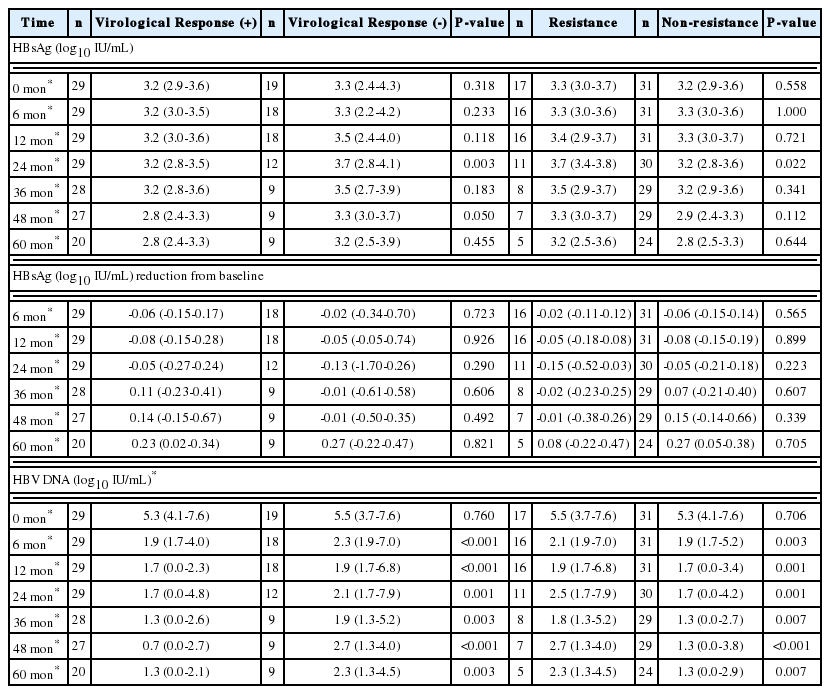
Twenty-nine patients (60.4%) achieved VR within 5 years. Treatment duration with initial nucleoside analogue was longer (66.0 months vs. 26.2 months, P<0.001) and incidence of resistance occurrence was lower (3.4% vs. 84.2%, P=0.001) in these patients. A tendency for ETV treatment was observed in the VR-positive group (27.6% vs. 5.3%, P=0.068). The gender composition (P=1.000), disease status (P=0.810), and number of HBeAg-positive patients (P=0.377) was not significantly different between two groups. Median age (P=0.134), baseline HBsAg level (P=0.318), HBV DNA level (P=0.760), AST (P=0.255), ALT (P=0.454), total bilirubin (P=0.204) and Child-Pugh score (P=0.377) were also similar between the groups (Table 2).
The median HBsAg level at each time point was similar between the groups except at 2 years; 3.2 vs. 3.7 log10 IU/mL at 2 years (P=0.003) (Table 3). The median decrease in HBsAg from baseline was not different between groups (Table 3). However, the median HBV DNA level at each time point was significantly different (Table 3).
Comparison of HBsAg level reduction according to the occurrence resistance
Seventeen patients (35.4%) experienced resistance to the antiviral agent during the treatment. Among these patients, one patient experienced VR within 5 years and subsequently developed resistance. Treatment duration with initial nucleoside analogue was shorter (26.2 months vs. 66.0 months, P<0.001) and achievement of VR within 5 years was lower (5.9% vs. 90.3%, P<0.001) in these patients. Use of LHV was more frequent proportion in the resistance group than that in the non-resistance group (100.0% vs. 71.0%, P=0.018). The incidence of male gender (P=0.486), disease status (P=0.757), and HBeAg-positive patients (P=0.764) was not significantly different between the groups. Median age (P=0.203), baseline HBsAg level (P=0.558), HBV DNA level (P=0.706), AST (P=0.880), ALT (P=0.948), total bilirubin (P=0.176), and Child-Pugh score (P=0.957) were also similar between the groups (Table 2).
Median HBsAg level at each time point was similar between the groups except at 2 years; 3.7 vs. 3.2 log10 IU/mL at 2 years (P=0.022) (Table 3). The median reduction in HBsAg from baseline was not different between the groups (Table 3). The median HBV DNA level at each time point was significantly different (Table 3).
Significant difference in HBsAg level from baseline
According to Friedman test, there was a statistically significant difference in HBsAg level between baseline and at 5 years treatment period in all patients (χ2=18.706, P=0.005). In subgroup analysis, VR-positive group (χ2=13.154, P=0.041), Non-resistance group (χ2=15.122, P=0.019) and LMV group (χ2=18.686, P=0.005) showed significant differences.
The difference in HBsAg level from baseline and each time point in all patients was not significant until year 3. A tendency for a difference was observed at year 4 (P=0.079), which became significant at year 5 (P=0.028). In a sub-analysis according to the experienced of flow the baseline VR within 5 years, the difference in HBsAg level terded to show at year 4 (P=0.072), and year 5 (P=0.075) in the VR-positive group. In a sub-analysis according to the development of resistance, the non-resistance group showed a significant difference in HBsAg level from the baseline at year 5 (P=0.041). In a sub-analysis according to the antiviral agent, the LMV group showed a significant difference at year 5 (P=0.041) (Fig. 1).
DISCUSSION
HBV infection is the one of the most common causes of chronic liver disease in Korea.7 Although the prevalence of chronic HBV infection is decreasing, it is still a major etiology of LC and HCC in Korea.8
Since the 1960s, HBsAg has been the hallmark of HBV infection.9 Studies that used newly available automated quantitative assays have shown that serum HBsAg levels vary significantly during the different phases of chronic HBV infection and are inversely correlated with the immune control of HBV; the higher the control, the lower the HBsAg level.10 Several studies have shown that the decline in serum HBsAg levels during treatment with peg-interferon mimics that of intrahepatic covalently closed circular DNA, suggesting that a decline in serum HBsAg level is associated with the induction of an effective anti-HBV immune response.11-13 In contrast, treatment with nucleoside analogues may induce pronounced HBV DNA declines; however, the effect on serum HBsAg level is very limited and the HBsAg decline during treatment is considerably slower than that observed for HBV DNA.3,14,15 Therefore, the clinical application of HBsAg level in patients treated with nucleoside analogues is limited.10
Many patients were treated with LMV in our study. Current treatment guidelines recommend ETV or tenofovir (TDF) as the first-line oral antiviral agent.16-18 However, ETV has been available since year 2007 in Korea and TDF has been available since 2011. Therefore, many patients who started before that time used LMV. Although LMV is less potent than ETV or TDF, we observed a reduction in HBsAg level if there was successful long-term treatment response. However, it is difficult to observe a significant reduction in HBsAg level with short-term treatment, although ETV is more potent than LMV. Reijinders et al compared HBsAg kinetics between a 48 week treatment with peg-interferon and ETV and observed a significant reduction in HBsAg level, mainly in peg-interferon-treated patients.14 Fung et al19 also reported that the majority of patients with CHB after 2 years of ETV treatment do not show a significant decline in HBsAg level despite suppression of HBV DNA. A successful long-term treatment is required to observe a significant change in HBsAg level with nucleoside analogues, and frequent HBsAg measurement may not be necessary.
In patients with CHB treated with LMV, the percentage of observed resistance is reported to be 24% if treated for >1 year and 70% if treated for >4 years.20 Fasano et al21 reported 11.7% HBsAg clearance in HBeAg-negative patients with CHB successfully treated with LMV monotherapy for at least 5 years. In that report, patients showing LMV resistance were censored. Therefore, the pattern of HBsAg level change in patients with resistance to LMV was not shown. In the current study, HBsAg level remained unchanged during the treatment in the resistance group. However, the reduction was significant after 5 years of treatment in patients without resistance. Furthermore, patients with a VR within 5 years did not show a difference in HBsAg, and one patient developed resistance after 5 years of treatment in the VR (+) group. This result shows that VR does not guarantee a patient to be free of resistance. Current CHB treatment guidelines suggest that a loss of HBsAg is the final treatment goal.16-18 HBsAg quantification may become more important than HBV DNA level in monitoring treatment efficacy.
Major limitation of current study is a small scale. We used non-parametric analysis for this reason. Although only difference of HBsAg level from baseline was significant, HBsAg level at each time point could become different between groups if more patients were included and follow up period was longer than 5 years. Difference of HBsAg level from baseline in VR-positive group was not significant, this may be also due to small number of patients.
In conclusion, a reduction in HBsAg level during nucleoside analogue treatment was not definite during the early phase, but it decreased slowly during treatment. Long-term treatment is required to observe significant reduction in HBsAg, but frequent HBsAg measurements are not necessary.
Notes
The authors have no conflicts to disclose.
Abbreviations
ALT
alanine aminotransferase
AST
aspartate aminotransferase
CHB
chronic hepatitis B
ETV
entecavir
HBeAg
hepatitis B 'e' antigen; HBsAg, hepatitis B surface antigen
HBV
hepatitis B virus
HCC
hepatocellular carcinoma
LC
liver cirrhosis
LMV
lamivudine
PCR
polymerase chain reaction
RFMP
restriction fragment mass polymorphism
TDF
tenofovir
VBT
virological breakthrough
VR
virological response