| Clin Mol Hepatol > Volume 30(2); 2024 > Article |
|
Dear Editor,
The consensus group, comprising patients from multiple societies, has updated the terminology for non-alcoholic fatty liver disease (NAFLD) to ŌĆ£metabolic dysfunction-associated steatotic liver diseaseŌĆØ (MASLD) to more accurately reflect the underlying pathophysiology [1]. This revision allows discernment of the underlying factors of steatotic liver disease (SLD) with increased precision and eliminates the potential for stigmatization [1,2]. Nonetheless, modifications to the terminology can result in misunderstanding and temporarily impede progress. In addition, to optimize the utilization of valuable research resources, it is imperative to build upon previous investigations on NAFLD and extend the studies to MASLD. In this regard, several studies have been conducted to accumulate evidence [3,4].
The etiological associations of hepatocellular carcinoma (HCC) have undergone a significant transformation in the past decade due to a marked decrease in active hepatitis C virus (HCV) infection. The increasing prevalence of NAFLD-related HCC has become a major public health issue worldwide [5,6]. In contrast to HCV-related HCC, the incidence of NAFLD-related HCC is relatively low. However, due to the significant number of individuals affected by NAFLD, challenges are posed from a cost-effective perspective to include and follow all patients to detect those affected at advanced stages [7]. Recent breakthroughs in systemic therapy for advanced HCC, such as the use of molecular-targeted agents and immune checkpoint inhibitors, e.g., atezolizumab in combination with bevacizumab (Atezo/Bev), have resulted in significantly improved prognoses for patients with these conditions. The impact of NAFLD on the prognosis of patients is a subject of ongoing discourse as the efficacy of therapeutic interventions may be influenced by both favorable and unfavorable factors related to the underlying etiologies [8,9]. Consequently, effective management and treatment of patients with HCC necessitates comprehension of the prognostic implications of NAFLD in such patients. However, no studies have investigated the relationship between NAFLD and MASLD in individuals with advanced HCC who have undergone Atezo/Bev therapy.
We aimed to compare the prognosis of unresectable HCC between patients with NAFLD and MASLD. This study included 216 consecutive patients with advanced HCC who received Atezo/Bev at one of 11 institutions in Japan between November 2020 and October 2023. All patients were of Asian origin. These participants were 78.7% male, median age 73 years, median body mass index 23.0 kg/m2, 98% performance status Ōēż1, 94% Child-Pugh class A, and median alpha-fetoprotein level of 42.2 ng/mL (Table 1). SLD was diagnosed based on the presence of moderate or severe hepatic steatosis on ultrasonography. The most common chemotherapies were first-line (136/216, 63.0%) followed by second-line (64/216, 29.6%).
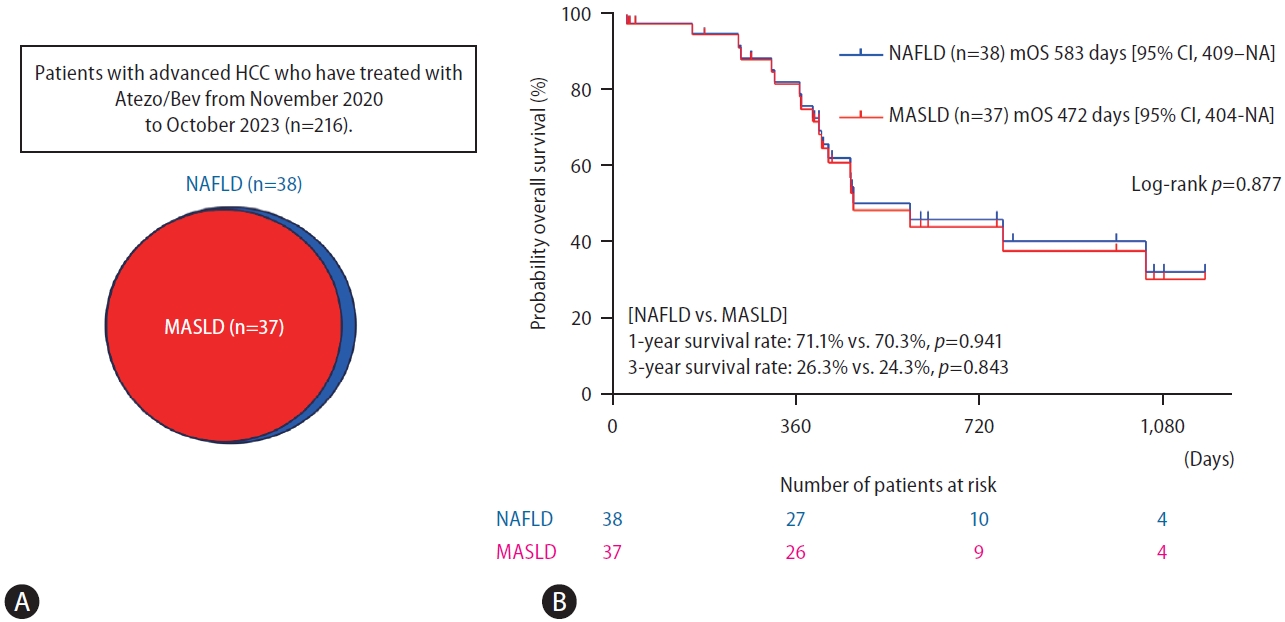
Of the 216 patients, 38 (17.6%) were diagnosed with NAFLD, including one patient who did not fulfill the cardiometabolic criteria for MASLD and was classified as cryptogenic SLD. (Fig. 1A). A significant proportion (97.4%) of the patients with NAFLD in our study were also diagnosed with MASLD. Thus, the backgrounds of the NAFLD and MASLD patients were nearly indistinguishable, a finding that is not surprising given that the difference was found only in one patient (Table 1). Our findings align well with previous studies reporting that more than 95% of NAFLD patients fulfilled the MASLD criteria [3,10]. Figure 1B shows the Kaplan-Meier curve for overall survival. No significant difference was observed in the overall survival rate between the NAFLD and MASLD groups (median overall survival in patients with NAFLD and MASLD was 583 days [95% certificate index, 409ŌĆōnon-applicable days] and 472 days [95% certificate index, 404ŌĆōnon-applicable days], respectively, P=0.877). The 1-year and 3-year survival rates were not significantly different between the patients with NAFLD and those with MASLD (1-year, 71.1% vs. 70.3%, P=0.941; 3-year, 26.3% vs. 24.3%, P=0.843) (Fig. 1B).
In conclusion, these results indicate that the prognosis for patients with advanced HCC undergoing Atezo/Bev treatment with MASLD is comparable to that in patients with NAFLD. It is crucial to validate these findings through an international cohort comprising a greater number of patients.
ACKNOWLEDGMENTS
This research was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP23fk0210090.
FOOTNOTES
AuthorsŌĆÖ contribution
Hiroyuki Suzuki: study concept, design, and statistical analysis; Shigeo Shimose: data extraction, interpretation of data, and critical revision of the manuscript; Hideki Iwamoto: interpretation of data, drafting, and critical revision of the manuscript; Takashi Niizeki: interpretation of data and critical revision of the manuscript; Takumi Kawaguchi: interpretation of data and critical revision of the manuscript.
Conflicts of Interest
Takumi Kawaguchi received lecture fees from Janssen Pharmaceutical K.K.; Taisho Pharmaceutical Co., Ltd.; Kowa Company, Ltd.; Otsuka Pharmaceutical Co., Ltd.; Eisai Co., Ltd.; ASKA Pharmaceutical Co., Ltd.; AbbVie GK; and EA Pharma Co., Ltd. The other authors declare no conflicts of interest.
Figure┬Ā1.
Prognosis of HCC under Atezo/Bev combination therapy in the NAFLD and MASLD groups. (A) The prevalence of NAFLD and MASLD in patients with HCC. (B) Probability of overall survival between the NAFLD and MASLD groups. HCC, hepatocellular carcinoma; Atezo/Bev, atezolizumab plus bevacizumab; NAFLD, nonalcoholic fatty liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease; NA, not applicable; mOS, median overall survival.
Table┬Ā1.
Baseline patients characteristics
REFERENCES
1. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol 2023;79:1542-1556.

2. Kim GA, Moon JH, Kim W. Critical appraisal of metabolic dysfunction-associated steatotic liver disease: Implication of Janus-faced modernity. Clin Mol Hepatol 2023;29:831-843.




3. Hagstr├Čm H, Vessby J, Ekstedt M, Shang Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J Hepatol 2024;80:e76-e77.
4. Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol 2024;80:e54-e56.


5. Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol 2023;79:516-537.


6. Tan DJH, Ng CH, Muthiah M, Yong JN, Chee D, Teng M, et al. Rising global burden of cancer attributable to high BMI from 2010 to 2019. Metabolism 2023;152:155744.


7. Reig M, Forner A, Rimola J, Ferrer-F├Ābrega J, Burrel M, GarciaCriado ├ü, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-693.



8. Pfister D, N├║├▒ez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021;592:450-456.


-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,287 View
- 90 Download
- ORCID iDs
-
Hiroyuki Suzuki

https://orcid.org/0000-0003-2383-5038 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



